The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis
- PMID: 14734524
- PMCID: PMC2211765
- DOI: 10.1084/jem.20031064
The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis
Abstract
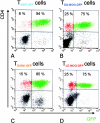
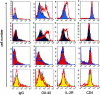
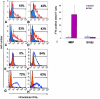
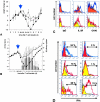
The clinical picture of experimental autoimmune encephalomyelitis (EAE) is critically dependent on the nature of the target autoantigen and the genetic background of the experimental animals. Potentially lethal EAE is mediated by myelin basic protein (MBP)-specific T cells in Lewis rats, whereas transfer of S100beta- or myelin oligodendrocyte glycoprotein (MOG)-specific T cells causes intense inflammatory response in the central nervous system (CNS) with minimal disease. However, in Dark Agouti rats, the pathogenicity of MOG-specific T cells resembles the one of MBP-specific T cells in the Lewis rat. Using retrovirally transduced green fluorescent T cells, we now report that differential disease activity reflects different levels of autoreactive effector T cell activation in their target tissue. Irrespective of their pathogenicity, the migratory activity, gene expression patterns, and immigration of green fluorescent protein(+) T cells into the CNS were similar. However, exclusively highly pathogenic T cells were significantly reactivated within the CNS. Without local effector T cell activation, production of monocyte chemoattractants was insufficient to initiate and propagate a full inflammatory response. Low-level reactivation of weakly pathogenic T cells was not due to anergy because these cells could be activated by specific antigen in situ as well as after isolation ex vivo.
Figures



References
-
- Wekerle, H., K. Kojima, J. Lannes-Vieira, H. Lassmann, and C. Linington. 1994. Animal models. Ann. Neurol. 36:S47–S53. - PubMed
-
- Ben-Nun, A., H. Wekerle, and I.R. Cohen. 1981. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur. J. Immunol. 11:195–199. - PubMed
-
- Benveniste, E.N. 1997. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J. Mol. Med. 75:165–173. - PubMed
-
- Kojima, K., H. Wekerle, H. Lassmann, T. Berger, and C. Linington. 1997. Induction of experimental autoimmune encephalomyelitis by CD4+ T cells specific for an astrocyte protein, S100β. J. Neural Transm. 49(Suppl.):43–51. - PubMed
-
- Linington, C., T. Berger, L. Perry, S. Weerth, D. Hinze-Selch, Y. Zhang, H.-C. Lu, H. Lassmann, and H. Wekerle. 1993. T cells specific for the myelin oligodendrocyte glycoprotein (MOG) mediate an unusual autoimmune inflammatory response in the central nervous system. Eur. J. Immunol. 23:1364–1372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

