Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization
- PMID: 14734525
- PMCID: PMC2211772
- DOI: 10.1084/jem.20031319
Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization
Abstract
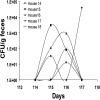
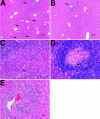
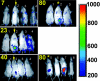
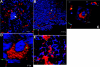
Host-adapted strains of Salmonella are capable of establishing a persistent infection in their host often in the absence of clinical disease. The mouse model of Salmonella infection has primarily been used as a model for the acute systemic disease. Therefore, the sites of long-term S. typhimurium persistence in the mouse are not known nor are the mechanisms of persistent infection clearly understood. Here, we show that S. typhimurium can persist for as long as 1 yr in the mesenteric lymph nodes (MLNs) of 129sv Nramp1(+)(/)(+) (Slc11a1(+)(/)(+)) mice despite the presence of high levels of anti-S. typhimurium antibody. Tissues from 129sv mice colonized for 60 d contain numerous inflammatory foci and lesions with features resembling S. typhi granulomas. Tissues from mice infected for 365 d have very few organized inflammatory lesions, but the bacteria continue to persist within macrophages in the MLN and the animals generally remain disease-free. Finally, chronically infected mice treated with an interferon-gamma neutralizing antibody exhibited symptoms of acute systemic infection, with evidence of high levels of bacterial replication in most tissues and high levels of fecal shedding. Thus, interferon-gamma, which may affect the level of macrophage activation, plays an essential role in the control of the persistent S. typhimurium infection in mice.
Figures



Similar articles
-
Mig-14 is an inner membrane-associated protein that promotes Salmonella typhimurium resistance to CRAMP, survival within activated macrophages and persistent infection.Mol Microbiol. 2005 Feb;55(3):954-72. doi: 10.1111/j.1365-2958.2004.04444.x. Mol Microbiol. 2005. PMID: 15661016
-
Host-pathogen interactions: Host resistance factor Nramp1 up-regulates the expression of Salmonella pathogenicity island-2 virulence genes.Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15705-10. doi: 10.1073/pnas.252415599. Epub 2002 Nov 19. Proc Natl Acad Sci U S A. 2002. PMID: 12441401 Free PMC article.
-
Salmonella Persistence and Host Immunity Are Dictated by the Anatomical Microenvironment.Infect Immun. 2020 Jul 21;88(8):e00026-20. doi: 10.1128/IAI.00026-20. Print 2020 Jul 21. Infect Immun. 2020. PMID: 32393507 Free PMC article.
-
Antigen-presenting cells and anti-Salmonella immunity.Microbes Infect. 2001 Nov-Dec;3(14-15):1239-48. doi: 10.1016/s1286-4579(01)01484-8. Microbes Infect. 2001. PMID: 11755412 Review.
-
How Pathogens Feel and Overcome Magnesium Limitation When in Host Tissues.Trends Microbiol. 2021 Feb;29(2):98-106. doi: 10.1016/j.tim.2020.07.003. Epub 2020 Aug 14. Trends Microbiol. 2021. PMID: 32807623 Free PMC article. Review.
Cited by
-
A Multi-Omic View of Host-Pathogen-Commensal Interplay in Salmonella-Mediated Intestinal Infection.PLoS One. 2013 Jun 26;8(6):e67155. doi: 10.1371/journal.pone.0067155. Print 2013. PLoS One. 2013. PMID: 23840608 Free PMC article.
-
HLA-B27 modulates intracellular growth of Salmonella pathogenicity island 2 mutants and production of cytokines in infected monocytic U937 cells.PLoS One. 2012;7(3):e34093. doi: 10.1371/journal.pone.0034093. Epub 2012 Mar 28. PLoS One. 2012. PMID: 22470519 Free PMC article.
-
Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota.Infect Immun. 2008 Jan;76(1):403-16. doi: 10.1128/IAI.01189-07. Epub 2007 Oct 29. Infect Immun. 2008. PMID: 17967858 Free PMC article.
-
Chronic Salmonella enterica serovar Typhimurium-induced colitis and cholangitis in streptomycin-pretreated Nramp1+/+ mice.Infect Immun. 2006 Sep;74(9):5047-57. doi: 10.1128/IAI.00072-06. Infect Immun. 2006. PMID: 16926396 Free PMC article.
-
Enterocyte-innate lymphoid cell crosstalk drives early IFN-γ-mediated control of Cryptosporidium.Mucosal Immunol. 2022 Feb;15(2):362-372. doi: 10.1038/s41385-021-00468-6. Epub 2021 Nov 8. Mucosal Immunol. 2022. PMID: 34750455 Free PMC article.
References
-
- Santos, R.L., S. Zhang, R.M. Tsolis, R.A. Kingsley, L.G. Adams, and A.J. Baumler. 2001. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 3:1335–1344. - PubMed
-
- Parkhill, J., G. Dougan, K.D. James, N.R. Thomson, D. Pickard, J. Wain, C. Churcher, K.L. Mungall, S.D. Bentley, M.T. Holden, et al. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature. 413:848–852. - PubMed
-
- Carter, P.B., and F.M. Collins. 1975. Peyer's patch responsiveness to Salmonella in mice. J. Reticuloendothel. Soc. 17:38–46. - PubMed
-
- Vasquez-Torres, A., J. Jones-Carson, A.J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W.T. Parks, and F. Fang. 1999. Extraintestinal dissemination of Salmonella via CD18-expressing phagocytes. Nature. 401:804–808. - PubMed
-
- House, D., A. Bishop, C. Parry, G. Dougan, and J. Wain. 2001. Typhoid fever: pathogenesis and disease. Curr. Opin. Infect. Dis. 14:573–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

