HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse
- PMID: 14734528
- PMCID: PMC2211771
- DOI: 10.1084/jem.20030648
HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse
Abstract
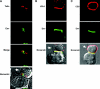
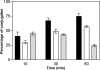
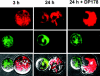
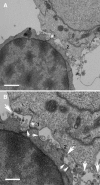
Direct cell-cell transfer is an efficient mechanism of viral dissemination within an infected host, and human immunodeficiency virus 1 (HIV-1) can exploit this mode of spread. Receptor recognition by HIV-1 occurs via interactions between the viral surface envelope glycoprotein (Env), gp120, and CD4 and a chemokine receptor, CCR5 or CXCR4. Here, we demonstrate that the binding of CXCR4-using HIV-1-infected effector T cells to primary CD4(+)/CXCR4(+) target T cells results in rapid recruitment to the interface of CD4, CXCR4, talin, and lymphocyte function-associated antigen 1 on the target cell, and of Env and Gag on the effector cell. Recruitment of these membrane molecules into polarized clusters was dependent on Env engagement of CD4 and CXCR4 and required remodelling of the actin cytoskeleton. Transfer of Gag from effector to target cell was observed by 1 h after conjugate formation, was independent of cell-cell fusion, and was probably mediated by directed virion fusion with the target cell. We propose that receptor engagement by Env directs the rapid, actin-dependent recruitment of HIV receptors and adhesion molecules to the interface, resulting in a stable adhesive junction across which HIV infects the target cell.
Figures






References
-
- Igakura, T., J. Stinchcombe, P. Goon, G. Taylor, J. Weber, G. Griffiths, Y. Tanaka, M. Osame, and C. Bangham. 2003. Spread of HTLV-1 between lymphocytes by virus-induced polarisation of the cytoskeleton. Science. 299:1713–1716. - PubMed
-
- Phillips, D. 1994. The role of cell-to-cell transmission in HIV infection. AIDS. 8:719–731. - PubMed
-
- Haase, A. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissue. Annu. Rev. Immunol. 17:625–656. - PubMed
-
- Sato, H., J. Orenstein, D. Dimitrov, and M. Martin. 1992. Cell-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology. 186:712–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

