A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules
- PMID: 14734535
- PMCID: PMC2172322
- DOI: 10.1083/jcb.200309096
A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules
Abstract
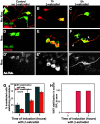
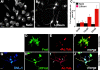
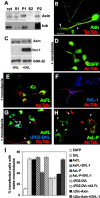
Dishevelled (DVL) is associated with axonal microtubules and regulates microtubule stability through the inhibition of the serine/threonine kinase, glycogen synthase kinase 3beta (GSK-3beta). In the canonical WNT pathway, the negative regulator Axin forms a complex with beta-catenin and GSK-3beta, resulting in beta-catenin degradation. Inhibition of GSK-3beta by DVL increases beta-catenin stability and TCF transcriptional activation. Here, we show that Axin associates with microtubules and unexpectedly stabilizes microtubules through DVL. In turn, DVL stabilizes microtubules by inhibiting GSK-3beta through a transcription- and beta-catenin-independent pathway. More importantly, axonal microtubules are stabilized after DVL localizes to axons. Increased microtubule stability is correlated with a decrease in GSK-3beta-mediated phosphorylation of MAP-1B. We propose a model in which Axin, through DVL, stabilizes microtubules by inhibiting a pool of GSK-3beta, resulting in local changes in the phosphorylation of cellular targets. Our data indicate a bifurcation in the so-called canonical WNT-signaling pathway to regulate microtubule stability.
Figures
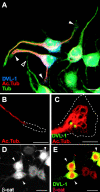
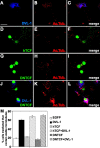
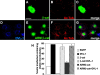
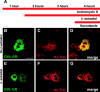

References
-
- Andersen, S.S., and G.Q. Bi. 2000. Axon formation: a molecular model for the generation of neuronal polarity. Bioessays. 22:172–179. - PubMed
-
- Arias, A.M., A.M. Brown, and K. Brennan. 1999. Wnt signalling: pathway or network? Curr. Opin. Genet. Dev. 9:447–454. - PubMed
-
- Baas, P.W. 1999. Microtubules and neuronal polarity: lessons from mitosis. Neuron. 22:23–31. - PubMed
-
- Behrens, J., B.A. Jerchow, M. Wurtele, J. Grimm, C. Asbrand, R. Wirtz, M. Kuhl, D. Wedlich, and W. Birchmeier. 1998. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science. 280:596–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

