Molecular dissection of water and glycerol permeability of the aquaglyceroporin from Plasmodium falciparum by mutational analysis
- PMID: 14734807
- PMCID: PMC337022
- DOI: 10.1073/pnas.0307295101
Molecular dissection of water and glycerol permeability of the aquaglyceroporin from Plasmodium falciparum by mutational analysis
Abstract
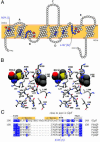
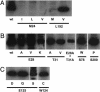
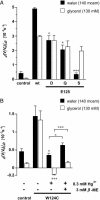
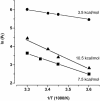
The selectivity of aquaporins for water and solutes is determined by pore diameter. Paradoxically, the wider pores of glycerol facilitators restrict water passage by an unknown mechanism. Earlier we characterized an aquaglyceroporin from Plasmodium falciparum with high permeability for both glycerol and water. We use point mutations to demonstrate that amino acids directly lining the pore are not responsible for the excellent water permeability of the Plasmodium aquaglyceroporin but affect permeability of pentitols. Within a conserved WET triad in the extracellular C-loop we identified a Plasmodium aquaglyceroporin-specific glutamate (E125) located in proximity to a conserved arginine (R196) at the pore mouth. Mutation of E125 to serine largely abolished water permeability. Concomitantly, the activation energy for water permeation was increased by 4 kcal/mol. Mutation of the adjacent tryptophan to cysteine led to irreversible inhibition of water passage by Hg(2+). This unequivocally proves the proximity of the couple W124/E125 close to the pore mouth. We conclude that in the Plasmodium aquaglyceroporin the electrostatic environment at the extracellular pore entry regulates water permeability.
Figures

References
-
- Borgnia, M., Nielsen, S., Engel, A. & Agre, P. (1999) Annu. Rev. Biochem. 68, 425–458. - PubMed
-
- Walz, T., Hirai, T., Murata, K., Heymann, J. B., Mitsuoka, K., Fujiyoshi, Y., Smith, B. L., Agre, P. & Engel, A. (1997) Nature 387, 624–627. - PubMed
-
- Cheng, A., van Hoek, A. N., Yeager, M., Verkman, A. S. & Mitra, A. K. (1997) Nature 387, 627–630. - PubMed
-
- Sui, H., Han, B. G., Lee, J. K., Walian, P. & Jap, B. K. (2001) Nature 414, 872–878. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

