Enhancement of cytotoxic T-lymphocyte responses in patients with gastrointestinal malignancies following vaccination with CEA peptide-pulsed dendritic cells
- PMID: 14735319
- PMCID: PMC11032759
- DOI: 10.1007/s00262-003-0491-7
Enhancement of cytotoxic T-lymphocyte responses in patients with gastrointestinal malignancies following vaccination with CEA peptide-pulsed dendritic cells
Abstract
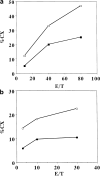
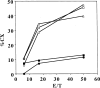
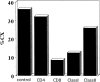
Carcinoembryonic antigen (CEA) is strongly expressed in a vast majority of gastrointestinal carcinomas. Recently, epitope peptides of CEA were identified. We have demonstrated HLA-A24-restricted peptide, CEA652[9] (TYACFVSNL), was capable of eliciting specific cytotoxic T lymphocytes (CTLs) which could lyse tumor cells expressing HLA-A24 and CEA. HLA-A24 is the most applicable MHC class I allele in the Japanese population. In this pilot study, we have used the peptide-pulsed dendritic cells (DCs) generated from peripheral blood mononuclear cells (PBMCs) supplemented with GM-CSF and IL-4 as the source of the vaccine. Eight patients with advanced CEA-expressing gastrointestinal malignancies received subcutaneous injections every 2 or 3 weeks. Immunomonitoring was performed by ELISpot (enzyme-linked immunosorbent spot) assay to measure the precursor frequency of CTLs and their capacity to elicit antitumor CTLs in vitro. Four of seven patients have developed their CTL response after vaccinations. DTH reaction was observed in one of eight patients at the DC-injected site. Skin biopsy at the injected site showed the infiltration of the lymphocytes. Furthermore, A24/CEA peptide tetramer assay revealed an increase in peptide-specific T-cell precursor frequency in vaccinated patients. No significant toxic adverse effects were observed, except for mild diarrhea in one case after three vaccinations. Three patients have shown stabilization of the disease after vaccinations. In conclusion, our results clearly demonstrated that our vaccination protocol was safe and might develop a CEA-specific CTL response in cancer patients.
Copyright 2004 Springer-Verlag
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

