The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb
- PMID: 14736846
- PMCID: PMC6729269
- DOI: 10.1523/JNEUROSCI.4002-03.2004
The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb
Abstract
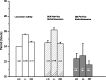
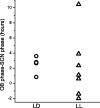
The suprachiasmatic nucleus (SCN) of the hypothalamus has been termed the master circadian pacemaker of mammals. Recent discoveries of damped circadian oscillators in other tissues have led to the hypothesis that the SCN synchronizes and sustains daily rhythms in these tissues. We studied the effects of constant lighting (LL) and of SCN lesions on behavioral rhythmicity and Period 1 (Per1) gene activity in the SCN and olfactory bulb (OB). We found that LL had similar effects on cyclic locomotor and feeding behaviors and Per1 expression in the SCN but had no effect on rhythmic Period 1 expression in the OB. LL lengthened the period of locomotor and SCN rhythms by approximately 1.6 hr. After 2 weeks in LL, nearly 35% of rats lost behavioral rhythmicity. Of these, 90% showed no rhythm in Per1-driven expression in their SCN. Returning the animals to constant darkness rapidly restored their daily cycles of running wheel activity and gene expression in the SCN. In contrast, the OB remained rhythmic with no significant change in period, even when cultured from animals that had been behaviorally arrhythmic for 1 month. Similarly, we found that lesions of the SCN abolished circadian rhythms in behavior but not in the OB. Together, these results suggest that LL causes the SCN to lose circadian rhythmicity and its ability to coordinate daily locomotor and feeding rhythms. The SCN, however, is not required to sustain all rhythms because the OB continues to oscillate in vivo when the SCN is arrhythmic or ablated.
Figures


References
-
- Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP (2002) Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol 12: 540-550. - PubMed
-
- Allada R, Emery P, Takahashi JS, Rosbash M (2001) Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci 24: 1091-1119. - PubMed
-
- Amir S, Cain S, Sullivan J, Robinson B, Stewart J (1999a) In rats, odor-induced Fos in the olfactory pathways depends on the phase of the circadian clock. Neurosci Lett 272: 175-178. - PubMed
-
- Amir S, Cain S, Sullivan J, Robinson B, Stewart J (1999b) Olfactory stimulation enhances light-induced phase shifts in free- running activity rhythms and Fos expression in the suprachiasmatic nucleus. Neuroscience 92: 1165-1170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
