JAMM: a metalloprotease-like zinc site in the proteasome and signalosome
- PMID: 14737182
- PMCID: PMC300881
- DOI: 10.1371/journal.pbio.0020002
JAMM: a metalloprotease-like zinc site in the proteasome and signalosome
Abstract
The JAMM (JAB1/MPN/Mov34 metalloenzyme) motif in Rpn11 and Csn5 underlies isopeptidase activities intrinsic to the proteasome and signalosome, respectively. We show here that the archaebacterial protein AfJAMM possesses the key features of a zinc metalloprotease, yet with a distinct fold. The histidine and aspartic acid of the conserved EX(n)HS/THX(7)SXXD motif coordinate a zinc, whereas the glutamic acid hydrogen-bonds an aqua ligand. By analogy to the active site of thermolysin, we predict that the glutamic acid serves as an acid-base catalyst and the second serine stabilizes a tetrahedral intermediate. Mutagenesis of Csn5 confirms these residues are required for Nedd8 isopeptidase activity. The active site-like architecture specified by the JAMM motif motivates structure-based approaches to the study of JAMM domain proteins and the development of therapeutic proteasome and signalosome inhibitors.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures

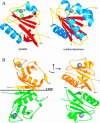

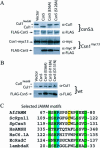
References
-
- Adams J. Development of the proteasome inhibitor PS-341. Oncologist. 2002;7:9–16. - PubMed
-
- Auld DS. Removal and replacement of metal-ions in metallopeptidases. Methods Enzymol. 1995;248:228–242. - PubMed
-
- Bartlett PA, Marlowe CK. Possible role for water dissociation in the slow binding of phosphorus-containing transition-state-analogue inhibitors of thermolysin. Biochemistry. 1987;26:8553–8561. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

