Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer
- PMID: 14737184
- PMCID: PMC314464
- DOI: 10.1371/journal.pbio.0020004
Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer
Abstract
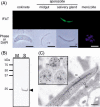
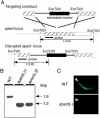
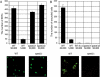
Liver infection is an obligatory step in malarial transmission, but it remains unclear how the sporozoites gain access to the hepatocytes, which are separated from the circulatory system by the liver sinusoidal cell layer. We found that a novel microneme protein, named sporozoite microneme protein essential for cell traversal (SPECT), is produced by the liver-infective sporozoite of the rodent malaria parasite, Plasmodium berghei. Targeted disruption of the spect gene greatly reduced sporozoite infectivity to the liver. In vitro cell invasion assays revealed that these disruptants can infect hepatocytes normally but completely lack their cell passage ability. Their apparent liver infectivity was, however, restored by depletion of Kupffer cells, hepatic macrophages included in the sinusoidal cell layer. These results show that malarial sporozoites access hepatocytes through the liver sinusoidal cell layer by cell traversal motility mediated by SPECT and strongly suggest that Kupffer cells are main routes for this passage. Our findings may open the way for novel malaria transmission-blocking strategies that target molecules involved in sporozoite migration to the hepatocyte.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures




References
-
- Bouwens L, Baekeland M, De Zanger R, Wisse E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology. 1986;6:718–722. - PubMed
-
- Carlton JM, Angiuoli SV, Suh BB, Kooij TW, Pertea M, et al. Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii . Nature. 2002;419:512–519. - PubMed
-
- Cerami C, Frevert U, Sinnis P, Takacs B, Clavijo P, et al. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell. 1992;70:1021–1033. - PubMed
-
- Hoffman SL, Franke ED, Hollingdale MR, Druihe P. Attacking the infected hepatocyte. In: Hoffman SL, editor. Malaria vaccine development. Washington, DC: American Society for Microbiology Press. pp. 1996:35–75.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

