HIV-1 Nef binds the DOCK2-ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis
- PMID: 14737186
- PMCID: PMC314466
- DOI: 10.1371/journal.pbio.0020006
HIV-1 Nef binds the DOCK2-ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis
Abstract
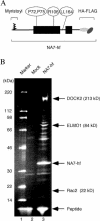
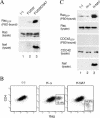
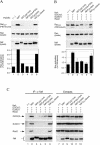
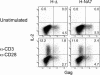
The infectious cycle of primate lentiviruses is intimately linked to interactions between cells of the immune system. Nef, a potent virulence factor, alters cellular environments to increase lentiviral replication in the host, yet the mechanisms underlying these effects have remained elusive. Since Nef likely functions as an adaptor protein, we exploited a proteomic approach to directly identify molecules that Nef targets to subvert the signaling machinery in T cells. We purified to near homogeneity a major Nef-associated protein complex from T cells and identified by mass spectroscopy its subunits as DOCK2-ELMO1, a key activator of Rac in antigen- and chemokine-initiated signaling pathways, and Rac. We show that Nef activates Rac in T cell lines and in primary T cells following infection with HIV-1 in the absence of antigenic stimuli. Nef activates Rac by binding the DOCK2-ELMO1 complex, and this interaction is linked to the abilities of Nef to inhibit chemotaxis and promote T cell activation. Our data indicate that Nef targets a critical switch that regulates Rac GTPases downstream of chemokine- and antigen-initiated signaling pathways. This interaction enables Nef to influence multiple aspects of T cell function and thus provides an important mechanism by which Nef impacts pathogenesis by primate lentiviruses.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures


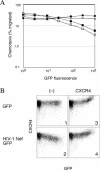
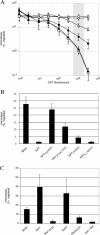

References
-
- Baur AS, Sawai ET, Dazin P, Fantl WJ, Cheng-Mayer C. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. - PubMed
-
- Baur AS, Sass G, Laffert B, Willbold D, Cheng-Mayer C, et al. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity. 1997;6:283–291. - PubMed
-
- Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, et al. Unconventional Rac-GEF activity is mediated through the Dock180–ELMO complex. Nat Cell Biol. 2002;4:574–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

