Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas
- PMID: 14737198
- PMCID: PMC314474
- DOI: 10.1371/journal.pbio.0020024
Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas
Abstract
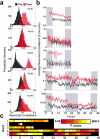
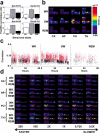
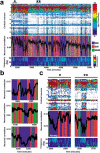
The discovery of experience-dependent brain reactivation during both slow-wave (SW) and rapid eye-movement (REM) sleep led to the notion that the consolidation of recently acquired memory traces requires neural replay during sleep. To date, however, several observations continue to undermine this hypothesis. To address some of these objections, we investigated the effects of a transient novel experience on the long-term evolution of ongoing neuronal activity in the rat forebrain. We observed that spatiotemporal patterns of neuronal ensemble activity originally produced by the tactile exploration of novel objects recurred for up to 48 h in the cerebral cortex, hippocampus, putamen, and thalamus. This novelty-induced recurrence was characterized by low but significant correlations values. Nearly identical results were found for neuronal activity sampled when animals were moving between objects without touching them. In contrast, negligible recurrence was observed for neuronal patterns obtained when animals explored a familiar environment. While the reverberation of past patterns of neuronal activity was strongest during SW sleep, waking was correlated with a decrease of neuronal reverberation. REM sleep showed more variable results across animals. In contrast with data from hippocampal place cells, we found no evidence of time compression or expansion of neuronal reverberation in any of the sampled forebrain areas. Our results indicate that persistent experience-dependent neuronal reverberation is a general property of multiple forebrain structures. It does not consist of an exact replay of previous activity, but instead it defines a mild and consistent bias towards salient neural ensemble firing patterns. These results are compatible with a slow and progressive process of memory consolidation, reflecting novelty-related neuronal ensemble relationships that seem to be context- rather than stimulus-specific. Based on our current and previous results, we propose that the two major phases of sleep play distinct and complementary roles in memory consolidation: pretranscriptional recall during SW sleep and transcriptional storage during REM sleep.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures



References
-
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: Theory and application to the study of motor units and physiological tremor. J Neurosci Methods. 1997;73:69–79. - PubMed
-
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. - PubMed
-
- Bryson D, Schacher S. Behavioral analysis of mammalian sleep and learning. Perspect Biol Med. 1969;13:71–79. - PubMed
-
- Cermak L, Craik F. Levels of processing in human memory. Indianapolis: John Wiley and Sons. 1979:479.
-
- Craik F, Lockhart R. Levels of processing: A framework for memory research. J Verb Learn Verb Behav. 1972;11:671–684.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

