Fluvastatin treatment inhibits leucocyte adhesion and extravasation in models of complement-mediated acute inflammation
- PMID: 14738444
- PMCID: PMC1808935
- DOI: 10.1111/j.1365-2249.2003.02358.x
Fluvastatin treatment inhibits leucocyte adhesion and extravasation in models of complement-mediated acute inflammation
Abstract
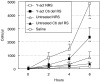
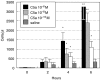
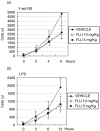
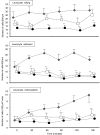
Complement activation plays a relevant role in the development of tissue damage under inflammatory conditions, and clinical and experimental observations emphasize its contribution to inflammatory vasculitides. Statins have recently been shown to reduce cardiovascular morbidity independently of plasma cholesterol lowering and in vitro studies support a direct anti-inflammatory action of these drugs. The aim of this study was to verify the in vivo effect of fluvastatin on complement-mediated acute peritoneal inflammation. The effect of oral treatment with fluvastatin was investigated in normo-cholesterolaemic rats that received intraperitoneal injection of either yeast-activated rat serum (Y-act RS) or lipopolysaccharide to induce peritoneal inflammation monitored by the number of PMN recruited in peritoneal fluid washes. In addition, vascular adherence and extravasation of leucocytes were evaluated by direct videomicroscopy examination on mesentery postcapillary venules topically exposed to Y-act RS. The number of PMN in the peritoneal washes of rats treated with fluvastatin was 38% lower than that of untreated animals (P < 0.05) 12 h after LPS injection, and was even lower (56%) in rats treated with Y-act RS already 8 h after injection (P < 0.02). Firm adhesion to endothelium and extravasation of leucocytes evaluated under direct videomicroscopy observation were significantly inhibited in fluvastatin treated rats (77% and 72%, respectively; P < 0.01), 120 min after treatment with Y-act RS. Our results demonstrate that fluvastatin inhibits in vivo complement-dependent acute peritoneal inflammation and suggest a role for statins in preventing the inflammatory flares usually associated with complement activation in chronic diseases, such as SLE or rheumatoid arthritis.
Figures
References
-
- Müller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–47. - PubMed
-
- Rus HG, Niculescu FI, Shin ML. Role of the C5b-9 complement complex in cell cycle and apoptosis. Immunol Rev. 2001;180:49–55. - PubMed
-
- Morgan BP, Walport MJ. Complement deficiency and disease. Immunol Today. 1991;12:301–6. - PubMed
-
- Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Adv Immunol. 1996;61:201–83. - PubMed
-
- Kirschfink M. Targeting complement in therapy. Immunol Rev. 2001;180:177–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

