Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis
- PMID: 14739341
- PMCID: PMC337053
- DOI: 10.1073/pnas.0308125100
Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis
Abstract
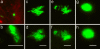
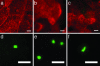
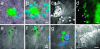
Uropathogenic Escherichia coli (UPEC) are capable of forming complex intracellular bacterial communities (IBC) within the superficial umbrella cells of the bladders of C3H and BALB/c mice. By using time-lapse fluorescence videomicroscopy to observe infected mouse bladder explants, we discovered that IBCs formed by uropathogenic E. coli progressed through four distinct developmental stages that differed with respect to growth rate, bacterial length, colony organization, motility, and its eventual dispersal. In the first phase, bacteria in the IBC were nonmotile, rod shaped, and grew rapidly in loosely organized colonies free in the cytoplasm of the bladder superficial umbrella cells. In the second phase, the loose collection of bacteria in the IBC matured into a slower growing, highly organized biofilm-like community consisting of coccoid bacteria that ultimately filled most of the cytoplasm. In the third phase, bacteria in the biofilm-like state in the IBC switched to a motile rod-shaped phenotype allowing detachment from the community and eventual fluxing out of the host cell. During the fourth phase, the bacteria filamented. Filamentation appeared to be in response to a Toll-like receptor 4-mediated innate defense mechanism. Bacteria that fluxed out of the superficial umbrella cells were able to reenter the IBC developmental cascade but with slower kinetics and ultimately a quiescent reservoir was established. Intracellular growth and filamentation provided an advantage to the bacteria in evading infiltrating polymorphonuclear leukocytes. This work has developed a technique to observe live infected organs and revealed a complex differentiation pathway that facilitates bacterial persistence in the urinary tract.
Figures



References
-
- Hooton, T. M. & Stamm, W. E. (1997) Infect. Dis. Clin. N. Am. 11, 551–581. - PubMed
-
- Svanborg, C. & Godaly, G. (1997) Infect. Dis. Clin. N. Am. 11, 513–529. - PubMed
-
- Foxman, B. (2002) Am. J. Med. 113, Suppl 1A, 5S–13S. - PubMed
-
- Mysorekar, I. U., Mulvey, M. A., Hultgren, S. J. & Gordon, J. I. (2002) J. Biol. Chem. 277, 7412–7419. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

