Treatment of advanced human immunodeficiency virus type 1 disease with the viral entry inhibitor PRO 542
- PMID: 14742190
- PMCID: PMC321533
- DOI: 10.1128/AAC.48.2.423-429.2004
Treatment of advanced human immunodeficiency virus type 1 disease with the viral entry inhibitor PRO 542
Abstract
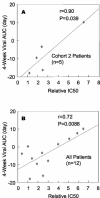
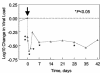
Viral entry inhibitors represent an emerging mode of therapy for human immunodeficiency virus type 1 (HIV-1) infection. PRO 542 (CD4-immunoglobulin G2) is a tetravalent CD4-immunoglobulin fusion protein that broadly neutralizes primary HIV-1 isolates. PRO 542 binds to the viral surface glycoprotein gp120 and blocks attachment and entry of virus into CD4(+) cells. Previously, PRO 542 demonstrated antiviral activity without significant toxicity when tested at single doses ranging to 10 mg/kg. In this study, 12 HIV-infected individuals were treated with 25-mg/kg single-dose PRO 542 and then monitored for safety, antiviral effects, and PRO 542 pharmacokinetics for 6 weeks. The study examined two treatment cohorts that differed in the extent of HIV-1 disease progression. PRO 542 at 25 mg/kg was well tolerated and demonstrated a serum half-life of 3 days. Statistically significant acute reductions in HIV-1 RNA levels were observed across all study patients, and greater antiviral effects were observed in the cohort of patients with more advanced HIV-1 disease. In advanced disease (HIV-1 RNA > 100,000 copies/ml; CD4 lymphocytes < 200 cells/mm(3)), PRO 542 mediated an 80% response rate and statistically significant approximately 0.5 log(10) mean reductions in viral load for 4 to 6 weeks posttreatment. Similar findings were obtained in an analysis of all (n = 11) advanced disease patients treated to date with single doses of PRO 542 ranging from 1 to 25 mg/kg. In addition, a significant correlation was observed between antiviral effects observed in vivo and viral susceptibility to PRO 542 in vitro. The findings support continued development of PRO 542 for salvage therapy of advanced HIV-1 disease.
Figures

References
-
- Allaway, G. P., K. L. Davis-Bruno, G. A. Beaudry, E. B. Garcia, E. L. Wong, A. M. Ryder, K. W. Hasel, M.-C. Gauduin, R. A. Koup, J. S. McDougal, and P. J. Maddon. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer which neutralizes primary HIV-1 isolates. AIDS Res. Hum. Retroviruses 11:533-539. - PubMed
-
- Berry, D. A., and B. W. Lindgren. 1996. Statistics: theory and methods. Wadsworth Publishing Company, Belmont, Calif.
-
- Binley, J. M., B. Clas, A. Gettie, M. Vesanen, D. C. Montefiori, L. Sawyer, J. Booth, M. Lewis, P. A. Marx, S. Bonhoeffer, and J. P. Moore. 2000. Passive infusion of immune serum into simian immunodeficiency virus-infected rhesus macaques undergoing a rapid disease course has minimal effect on plasma viremia. Virology 270:237-249. - PubMed
-
- Biscone, M. J., T. C. Pierson, and R. W. Doms. 2002. Opportunities and challenges in targeting HIV entry. Curr. Opin. Pharmacol. 2:529-533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

