Pharmacokinetics of saquinavir plus low-dose ritonavir in human immunodeficiency virus-infected pregnant women
- PMID: 14742191
- PMCID: PMC321538
- DOI: 10.1128/AAC.48.2.430-436.2004
Pharmacokinetics of saquinavir plus low-dose ritonavir in human immunodeficiency virus-infected pregnant women
Abstract
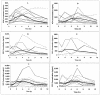
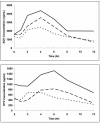
The physiologic changes that occur during pregnancy make it difficult to predict antiretroviral pharmacokinetics (PKs), but few data exist on the PKs of protease inhibitors in human immunodeficiency virus (HIV)-infected pregnant women. The objective of the present study was to determine the PKs of ritonavir (RTV)-enhanced saquinavir (SQV) in HIV-infected pregnant women by an area under the curve (AUC)-targeted approach. A phase I, formal PK evaluation was conducted with HIV-infected pregnant woman during gestation, during labor and delivery, and at 6 weeks postpartum. The SQV-RTV regimen was 800/100 mg twice a day (b.i.d.), and nucleoside analogs were administered concomitantly. The SQV exposure targeted was an AUC at 24 h of 10,000 ng. h/ml. Participants were evaluated for 12-h steady-state PKs at each time period. Thirteen subjects completed the PK evaluations during gestation, 7 completed the PK evaluations at labor and delivery, and 12 completed the PK evaluations postpartum. The mean baseline weight was 67.4 kg, and the median length of gestation was 23.3 weeks. All subjects achieved SQV exposures in excess of the target AUC. The SQV AUCs at 12 h (AUC(12)s) during gestation (29,373 +/- 17,524 ng. h/ml [mean +/- standard deviation]), during labor and delivery (26,189 +/- 22,138 ng. h/ml), and during the postpartum period (35,376 +/- 26,379 ng. h/ml) were not significantly different. The mean values of the PK parameters for RTV were lower during gestation than during the postpartum period: for AUC(12), 7,811 and 13,127 ng. h/ml, respectively; for trough concentrations, 376 and 632 ng/ml, respectively; and for maximum concentrations, 1,256 and 2,252 ng/ml, respectively (P </= 0.05 for all comparisons). This is the first formal PK evaluation of a dual protease inhibitor regimen with HIV-infected pregnant women. The level of SQV exposure was sufficient at each time of evaluation. These data demonstrate large variability in SQV and RTV concentrations and suggest that RTV concentrations are altered by pregnancy. These PK results suggest that SQV-RTV at 800/100 mg b.i.d. appears to be a reasonable treatment option for this population.
Figures
References
-
- Acosta, E. P., T. N. Kakuda, R. C. Brundage, P. L. Anderson, and C. V. Fletcher. 2000. Pharmacodynamics of HIV-1 protease inhibitors. Clin. Infect. Dis. 30(Suppl. 2):S151-S159. - PubMed
-
- Acosta, E. P., C. D. Zorrilla, R. Van Dyke, A. Bardeguez, B. Smith, M. Hughes, J. Pitt, H. Watts, L. Mofenson, and the Pediatric AIDS Clinical Trials Group 386 Protocol Team. 2001. Pharmacokinetics of saquinavir-SGC in HIV-infected pregnant women. HIV Clin. Trials 2:460-465. - PubMed
-
- Acosta, E. P., J. G. Gerber, and the Adult ACTG Pharmacology Committee. 2002. Position paper on therapeutic drug monitoring of antiretroviral agents. AIDS Res. Hum. Retrovir. 18:825-834. - PubMed
-
- Audus, K. L., M. L. Soares, and J. S. Hunt. 2002. Characteristics of the fetal/maternal interface with potential usefulness in the development of future immunological and pharmacological strategies. J. Pharmacol. Exp. Ther. 301:402-409. - PubMed
-
- Condra, J. H., C. J. Petropoulos, R. Ziermann, W. A. Schleif, M. Shivaprakash, and E. A. Emini. 2000. Drug resistance and predicted virologic responses to human immunodeficiency virus type 1 protease inhibitor therapy. J. Infect. Dis. 182:758-765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

