New class of bacterial phenylalanyl-tRNA synthetase inhibitors with high potency and broad-spectrum activity
- PMID: 14742205
- PMCID: PMC321521
- DOI: 10.1128/AAC.48.2.525-532.2004
New class of bacterial phenylalanyl-tRNA synthetase inhibitors with high potency and broad-spectrum activity
Abstract
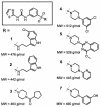
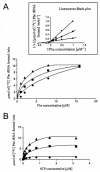
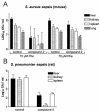
Phenylalanyl (Phe)-tRNA synthetase (Phe-RS) is an essential enzyme which catalyzes the transfer of phenylalanine to the Phe-specific transfer RNA (tRNA(Phe)), a key step in protein biosynthesis. Phenyl-thiazolylurea-sulfonamides were identified as a novel class of potent inhibitors of bacterial Phe-RS by high-throughput screening and chemical variation of the screening hit. The compounds inhibit Phe-RS of Escherichia coli, Haemophilus influenzae, Streptococcus pneumoniae, and Staphylococcus aureus, with 50% inhibitory concentrations in the nanomolar range. Enzyme kinetic measurements demonstrated that the compounds bind competitively with respect to the natural substrate Phe. All derivatives are highly selective for the bacterial Phe-RS versus the corresponding mammalian cytoplasmic and human mitochondrial enzymes. Phenyl-thiazolylurea-sulfonamides displayed good in vitro activity against Staphylococcus, Streptococcus, Haemophilus, and Moraxella strains, reaching MICs below 1 micro g/ml. The antibacterial activity was partly antagonized by increasing concentrations of Phe in the culture broth in accordance with the competitive binding mode. Further evidence that inhibition of tRNA(Phe) charging is the antibacterial principle of this compound class was obtained by proteome analysis of Bacillus subtilis. Here, the phenyl-thiazolylurea-sulfonamides induced a protein pattern indicative of the stringent response. In addition, an E. coli strain carrying a relA mutation and defective in stringent response was more susceptible than its isogenic relA(+) parent strain. In vivo efficacy was investigated in a murine S. aureus sepsis model and a S. pneumoniae sepsis model in rats. Treatment with the phenyl-thiazolylurea-sulfonamides reduced the bacterial titer in various organs by up to 3 log units, supporting the potential value of Phe-RS as a target in antibacterial therapy.
Figures


References
-
- Anderson, R. T., Jr., and D. V. Santi. 1976. Phenylalanyl transfer ribonucleic acid synthetase from Escherichia coli B. Potent inhibition by analogues of N-benzyl-2-phenylethylamine. J. Med. Chem. 19:1270-1275. - PubMed
-
- Appelbaum, P. C. 2002. Resistance among Streptococcus pneumoniae: implications for drug selection. Clin. Infect. Dis. 34:1613-1620. - PubMed
-
- Baines, P. J., D. Jackson, G. Mellows, A. J. Swaisland, and T. C. G. Tasker. 1984. Mupirocin: its chemistry and metabolism. R. Soc. Med. Int. Congr. Symp. Ser. 80:13-22.
-
- Baltzinger, M., and E. Holler. 1982. Catalytic mechanism of phenylalanyl-tRNA synthetase of Escherichia coli K10. Conformational change and tRNAPhe phenylalanylation are concerted. Biochemistry 21:2467-2476. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

