Enhanced saquinavir exposure in human immunodeficiency virus type 1-infected patients with diarrhea and/or wasting syndrome
- PMID: 14742207
- PMCID: PMC321510
- DOI: 10.1128/AAC.48.2.538-545.2004
Enhanced saquinavir exposure in human immunodeficiency virus type 1-infected patients with diarrhea and/or wasting syndrome
Abstract
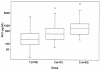
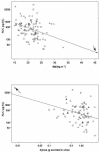
The protease inhibitor saquinavir was administered to 100 human immunodeficiency virus type 1 (HIV-1)-infected patients as a single 600-mg oral dose (hard gelatin capsules) with a standard breakfast, including 200 ml of grapefruit juice, during an open-label trial to assess whether diarrhea and/or wasting syndrome has consequences on its pharmacokinetics. Three groups of patients were enrolled: group 1, asymptomatic patients (n = 30); group 2, AIDS symptomatic patients without body weight loss or diarrhea (n = 37); and group 3, AIDS symptomatic patients with severe body weight loss and/or diarrhea (n = 33). Clinical and biological data (covariates) were collected. A population approach was performed with three blood samples per patient to estimate the mean population pharmacokinetic parameters (clearance [CL]/oral bioavailability [F], V/F, k(a), and lag time) and the derived ones (k(el), C(max), T(max), and area under the curve [AUC]). The relationships between groups, exposure (i.e., estimated individual post hoc AUCs), and covariates were explored by using multiple linear regressions. A significant increase in median AUCs (165, 349, and 705 ng. h. ml(-1) for groups 1, 2, and 3, respectively [P < 0.0001]) was observed. The enhancement in saquinavir exposure could be due to the destruction of the transporters in enterocytes and/or to the enlargement of their tight junctions, allowing a paracellular crossing of saquinavir as the illness spreads. Because of grapefruit juice intake by every patient, no implication of CYP3A4 could be assessed. These results strongly suggest that, despite its low intrinsic oral bioavailability, saquinavir can be considered as a relevant treatment for HIV-1-infected patients with diarrhea and/or wasting syndrome. This must be evaluated in a long-term period.
Figures

References
-
- Akinbami, F. O., G. A. Brown, and A. S. McNeish. 1989. Intestinal permeability as a measure of small mucosal integrity: correlation with jejunal biopsy. Afr. J. Med. Med. Sci. 18:187-192. - PubMed
-
- Alsenz, J., H. Steffen, and R. Alex. 1998. Active apical secretory efflux of the HIV protease inhibitors saquinavir and ritonavir in Caco-2 cell monolayers. Pharm. Res. 15:423-428. - PubMed
-
- Barry, M., S. Gibbons, D. Back, and F. Mulcahy. 1997. Protease inhibitors in patients with AIDS disease: clinically important pharmacokinetic considerations. Clin. Pharmacokinet. 32:194-209. - PubMed
-
- Benet, L. Z., T. Izumi, Y. Zhang, J. A. Silverman, and V. J. Wacher. 1999. Intestinal MDR transport proteins and P-450 enzymes as barriers to oral drug delivery. J. Control Release 62:25-31. - PubMed
-
- Bobin, S., D. Bouhour, S. Durupt, A. Boibieux, V. Girault, and D. Peyramond. 1998. Importance of antiproteases in the treatment of microsporidia and/or cryptosporidia infections in HIV-seropositive patients. Pathol. Biol. 46:418-419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

