Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium
- PMID: 14742263
- PMCID: PMC1602270
- DOI: 10.1016/S0002-9440(10)63147-1
Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium
Abstract
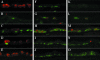
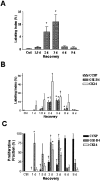
Commitment of the pulmonary epithelium to bronchial and bronchiolar airway lineages occurs during the transition from pseudoglandular to cannalicular phases of lung development, suggesting that regional differences exist with respect to the identity of stem and progenitor cells that contribute to epithelial maintenance in adulthood. We previously defined a critical role for Clara cell secretory protein-expressing (CE) cells in renewal of bronchiolar airway epithelium following injury. Even though CE cells are also the principal progenitor for maintenance of the bronchial airway epithelium, CE cell injury is resolved through a mechanism involving recruitment of a second progenitor cell population that we now identify as a GSI-B(4) reactive, cytokeratin-14-expressing basal cell. These cells exhibit multipotent differentiation capacity as assessed by analysis of cellular phenotype within clones of LacZ-tagged cells. Clones were derived from K14-expressing cells tagged in a cell-type-specific fashion by ligand-regulable Cre recombinase-mediated genomic rearrangement of the ROSA26 recombination substrate allele. We conclude that basal cells represent an alternative multipotent progenitor cell population of bronchial airways and that progenitor cell selection is dictated by the type of airway injury.
Figures




References
-
- Shannon JM. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev Biol. 1994;166:600–614. - PubMed
-
- Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. - PubMed
-
- Evans MJ, Moller PC. Biology of airway basal cells. Exp Lung Res. 1991;17:513–531. - PubMed
-
- Inayama Y, Hook GER, Brody AR, Cameron GS, Jetten AM, Gilmore LB, Gray T, Nettesheim P. The differentiation potential of tracheal basal cells. Lab Invest. 1988;58:706–717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

