Candida albicans yeast and germ tube forms interfere differently with human monocyte differentiation into dendritic cells: a novel dimorphism-dependent mechanism to escape the host's immune response
- PMID: 14742527
- PMCID: PMC321580
- DOI: 10.1128/IAI.72.2.833-843.2004
Candida albicans yeast and germ tube forms interfere differently with human monocyte differentiation into dendritic cells: a novel dimorphism-dependent mechanism to escape the host's immune response
Abstract
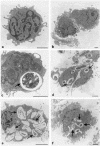
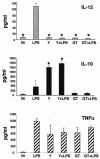
The ability of Candida albicans to convert from the yeast (Y) form to mycelial forms through germ tube (GT) formation is considered a key feature of the transition of the organism from commensalism to virulence. We show here that human monocytes cultured with granulocyte-macrophage colony-stimulating factor and interleukin-4 (IL-4) after phagocytosis of Y forms did not differentiate into dendritic cells (DCs); they retained CD14, did not acquire CD1a, and were unable to express the maturation markers CD83 and CCR7. Moreover, they did not produce IL-12p70 but secreted IL-10. In addition, they spontaneously expressed high levels of tumor necrosis factor alpha (TNF-alpha), IL-6, and IL-8 mRNA transcripts and were able to induce proliferation of alloreactive memory but not naïve T lymphocytes. Conversely, monocytes that had phagocytosed GT forms differentiated into mature CD83+ and CCR7+ DCs; however, there was no up-regulation of CD40, CD80, and major histocompatibility complex class II, irrespective of lipopolysaccharide (LPS) treatment. In addition, these cells were unable to produce IL-12 even after LPS stimulation, but they were not functionally exhausted, as shown by their capacity to express TNF-alpha and IL-8 mRNA transcripts. These cells were able to prime naïve T cells but not to induce their functional polarization into effector cells. These data indicate that phagocytosis of Y and GT forms has profound and distinct effects on the differentiation pathway of monocytes. Thus, the differentiation of human monocytes into DCs appears to be tunable and exploitable by C. albicans to elude immune surveillance.
Figures



References
-
- Allavena, P., L. Piemonti, D. Longoni, S. Bernasconi, A. Stoppacciaro, L. Ruco, and A. Mantovani. 1998. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur. J. Immunol. 28:359-369. - PubMed
-
- Bistoni, F., E. Cenci, A. Mencacci, E. Schiaffella, P. Mosci, P. Puccetti, and L. Romani. 1993. Mucosal and systemic T helper cell function after intragastric colonization of adult mice with Candida albicans. J. Infect. Dis. 168:1449-1457. - PubMed
-
- Cassone, A., F. De Bernardis, C. M. Ausiello, M. J. Gomez, M. Boccanera, R. La Valle, and A. Torosantucci. 1998. Immunogenic and protective Candida albicans constituents. Res. Immunol 149:289-299. (Discussion, 149:504.) - PubMed
-
- Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, I. Durand, M. Cella, A. Lanzavecchia, and J. Banchereau. 1997. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha. II. Functional analysis. Blood 90:1458-1470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

