Haemophilus influenzae porin induces Toll-like receptor 2-mediated cytokine production in human monocytes and mouse macrophages
- PMID: 14742577
- PMCID: PMC321594
- DOI: 10.1128/IAI.72.2.1204-1209.2004
Haemophilus influenzae porin induces Toll-like receptor 2-mediated cytokine production in human monocytes and mouse macrophages
Abstract
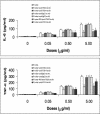
The production of proinflammatory cytokines is likely to play a major pathophysiological role in meningitis and other infections caused by Haemophilus influenzae type b (Hib). Previous studies have shown that Hib porin contributes to signaling of the inflammatory cascade. We examined here the role of Toll-like receptors (TLRs) and the TLR-associated adaptor protein MyD88 in Hib porin-induced production of tumor necrosis factor alpha (TNF-alpha) and interleukin-6 (IL-6). Hib porin-induced TNF-alpha and IL-6 production was virtually eliminated in macrophages from TLR2- or MyD88-deficient mice. In contrast, macrophages from lipopolysaccharide (LPS)-hyporesponsive C3H/HeJ mice, which are defective in TLR4 function, responded normally to Hib porin. Moreover anti-TLR2 antibodies but not anti-TLR4 antibodies significantly reduced Hib porin-stimulated TNF-alpha and IL-6 release from the human monocytic cell line THP-1. These data indicate that the TLR2/MyD88 pathway plays an essential role in Hib porin-mediated cytokine production. These findings may be useful in the development of alternative therapies aimed at reducing excessive inflammatory responses during Hib infections.
Figures



References
-
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. - PubMed
-
- Aliprantis, A. O., R. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. - PubMed
-
- Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, et al. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. - PubMed
-
- Cerami, A. 1992. Inflammatory cytokines. Clin. Immunol. Immunopathol. 62:3-10. - PubMed
-
- Diab, A., J. Zhu, L. Lindquist, B. Wretlind, H. Link, and M. Bakhiet. 1997. Cytokine mRNA profiles during the course of experimental Haemophilus influenzae bacterial meningitis. Clin. Immunol. Immunopathol. 85:236-452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

