NO66, a highly conserved dual location protein in the nucleolus and in a special type of synchronously replicating chromatin
- PMID: 14742713
- PMCID: PMC379278
- DOI: 10.1091/mbc.e03-08-0623
NO66, a highly conserved dual location protein in the nucleolus and in a special type of synchronously replicating chromatin
Abstract
It has recently become clear that the nucleolus, the most prominent nuclear subcompartment, harbors diverse functions beyond its classic role in ribosome biogenesis. To gain insight into nucleolar functions, we have purified amplified nucleoli from Xenopus laevis oocytes using a novel approach involving fluorescence-activated cell sorting techniques. The resulting protein fraction was analyzed by mass spectrometry and used for the generation of monoclonal antibodies directed against nucleolar components. Here, we report the identification and molecular characterization of a novel, ubiquitous protein, which in most cell types appears to be a constitutive nucleolar component. Immunolocalization studies have revealed that this protein, termed NO66, is highly conserved during evolution and shows in most cells analyzed a dual localization pattern, i.e., a strong enrichment in the granular part of nucleoli and in distinct nucleoplasmic entities. Colocalizations with proteins Ki-67, HP1alpha, and PCNA, respectively, have further shown that the staining pattern of NO66 overlaps with certain clusters of late replicating chromatin. Biochemical experiments have revealed that protein NO66 cofractionates with large preribosomal particles but is absent from cytoplasmic ribosomes. We propose that in addition to its role in ribosome biogenesis protein NO66 has functions in the replication or remodeling of certain heterochromatic regions.
Figures
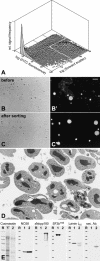
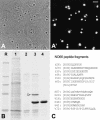
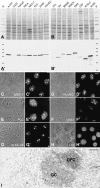
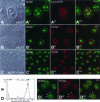




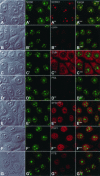
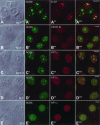
Similar articles
-
Protein NO52--a constitutive nucleolar component sharing high sequence homologies to protein NO66.Eur J Cell Biol. 2005 Mar;84(2-3):279-94. doi: 10.1016/j.ejcb.2004.12.022. Eur J Cell Biol. 2005. PMID: 15819408
-
A novel helicase-type protein in the nucleolus: protein NOH61.Mol Biol Cell. 2000 Apr;11(4):1153-67. doi: 10.1091/mbc.11.4.1153. Mol Biol Cell. 2000. PMID: 10749921 Free PMC article.
-
Requirement of the protein B23 for nucleolar disassembly induced by the FRGY2a family proteins.J Biol Chem. 2006 Mar 24;281(12):8153-60. doi: 10.1074/jbc.M512890200. Epub 2006 Jan 16. J Biol Chem. 2006. PMID: 16415342 Free PMC article.
-
The functional organization of the nucleolus in proliferating plant cells.Eur J Histochem. 2000;44(2):117-31. Eur J Histochem. 2000. PMID: 10968360 Review.
-
Nucleoli in embryos: a central structural platform for embryonic chromatin remodeling?Chromosome Res. 2019 Mar;27(1-2):129-140. doi: 10.1007/s10577-018-9590-3. Epub 2018 Nov 8. Chromosome Res. 2019. PMID: 30406864 Review.
Cited by
-
To Erase or Not to Erase: Non-Canonical Catalytic Functions and Non-Catalytic Functions of Members of Histone Lysine Demethylase Families.Int J Mol Sci. 2024 Jun 24;25(13):6900. doi: 10.3390/ijms25136900. Int J Mol Sci. 2024. PMID: 39000010 Free PMC article. Review.
-
Current understanding of mdig/MINA in human cancers.Genes Cancer. 2015 Jul;6(7-8):288-302. doi: 10.18632/genesandcancer.73. Genes Cancer. 2015. PMID: 26413213 Free PMC article. Review.
-
Modifying the maker: Oxygenases target ribosome biology.Translation (Austin). 2015 Feb 3;3(1):e1009331. doi: 10.1080/21690731.2015.1009331. eCollection 2015 Jan-Jun. Translation (Austin). 2015. PMID: 26779412 Free PMC article.
-
Structural insights into histone demethylase NO66 in interaction with osteoblast-specific transcription factor osterix and gene repression.J Biol Chem. 2013 Jun 7;288(23):16430-16437. doi: 10.1074/jbc.M112.446849. Epub 2013 Apr 24. J Biol Chem. 2013. PMID: 23620590 Free PMC article.
-
Origin and evolution of peptide-modifying dioxygenases and identification of the wybutosine hydroxylase/hydroperoxidase.Nucleic Acids Res. 2010 Sep;38(16):5261-79. doi: 10.1093/nar/gkq265. Epub 2010 Apr 27. Nucleic Acids Res. 2010. PMID: 20423905 Free PMC article.
References
-
- Andersen, J.S., Lyon, C.E., Fox, A.H., Leung, A.K., Lam, Y.W., Stehen, H., Mann, M., and Lamond, A.I. (2002). Directed proteomic analysis of the human nucleolus. Curr. Biol. 12, 1-11. - PubMed
-
- Azum-Gélade, M.C., Noaillac-Depeyre, J., Caizergues-Ferrer, M., and Gas, N. (1994). Cell cycle redistribution of U3 snRNA and fibrillarin. Presence in the cytoplasmic nucleolus remnant and in the prenucleolar bodies at telophase. J. Cell Sci. 107, 463-475. - PubMed
-
- Baynton, K., Otterlei, M., Bjoras, M., von Kobbe, C., Bohr, V.A., and Seeberg, E. (2003). WRN interacts physically and functionally with the recombination mediator protein RAD52. J. Biol. Chem. 278, 36476-36486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

