Syntaxin-6 SNARE involvement in secretory and endocytic pathways of cultured pancreatic beta-cells
- PMID: 14742717
- PMCID: PMC379267
- DOI: 10.1091/mbc.e03-08-0554
Syntaxin-6 SNARE involvement in secretory and endocytic pathways of cultured pancreatic beta-cells
Abstract
In pancreatic beta-cells, the syntaxin 6 (Syn6) soluble N-ethylmaleimide-sensitive factor attachment protein receptor is distributed in the trans-Golgi network (TGN) (with spillover into immature secretory granules) and endosomes. A possible Syn6 requirement has been suggested in secretory granule biogenesis, but the role of Syn6 in live regulated secretory cells remains unexplored. We have created an ecdysone-inducible gene expression system in the INS-1 beta-cell line and find that induced expression of a membrane-anchorless, cytosolic Syn6 (called Syn6t), but not full-length Syn6, causes a prominent defect in endosomal delivery to lysosomes, and the TGN, in these cells. The defect occurs downstream of the endosomal branchpoint involved in transferrin recycling, and upstream of the steady-state distribution of mannose 6-phosphate receptors. By contrast, neither acquisition of stimulus competence nor the ultimate size of beta-granules is affected. Biosynthetic effects of dominant-interfering Syn6 seem limited to slowed intragranular processing to insulin (achieving normal levels within 2 h) and minor perturbation of sorting of newly synthesized lysosomal proenzymes. We conclude that expression of the Syn6t mutant slows a rate-limiting step in endosomal maturation but provides only modest and potentially indirect interference with regulated and constitutive secretory pathways, and in TGN sorting of lysosomal enzymes.
Figures
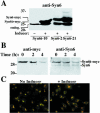








 : Syn 6-21 cells or Syn6t-10 cells were transfected with a plasmid encoding Alb-CPD and either uninduced (-) or induced for 24 h with ponasterone (+) before a 2-h continuous uptake with an anti-albumin polyclonal antibody (in red) as well as the immunofluorescent distribution of TGN38 by using a mAb (in green). In cells expressing Syn6t, transfected cells are marked with small arrows. Experiment
: Syn 6-21 cells or Syn6t-10 cells were transfected with a plasmid encoding Alb-CPD and either uninduced (-) or induced for 24 h with ponasterone (+) before a 2-h continuous uptake with an anti-albumin polyclonal antibody (in red) as well as the immunofluorescent distribution of TGN38 by using a mAb (in green). In cells expressing Syn6t, transfected cells are marked with small arrows. Experiment  : INS cells were transfected with Alb-CPD, full-length Syn6, or Syn6t-encoding plasmids, alone or in combination as indicated (double transfections used a 5:1 ratio of syntaxin DNA to Alb-CPD DNA). The cells were then labeled as in experiment
: INS cells were transfected with Alb-CPD, full-length Syn6, or Syn6t-encoding plasmids, alone or in combination as indicated (double transfections used a 5:1 ratio of syntaxin DNA to Alb-CPD DNA). The cells were then labeled as in experiment  . In cells expressing Syn6t, transfected cells are marked with small arrows. Experiment
. In cells expressing Syn6t, transfected cells are marked with small arrows. Experiment  : Syn 6-21 cells or Syn6t-1 cells were transfected with a plasmid encoding Tac-TGN38 and either uninduced (-) or induced for 24 h with ponasterone (+) before a 2-h continuous uptake with a mAb anti-Tac (in red) as well as the immunofluorescent distribution by using a polyclonal anti-TGN38 lumenal domain (in green). In the latter two panels, the nuclei are also stained with 4,6-diamidino-2-phenylindole (in blue). Transfected cells are marked with arrows. Experiment
: Syn 6-21 cells or Syn6t-1 cells were transfected with a plasmid encoding Tac-TGN38 and either uninduced (-) or induced for 24 h with ponasterone (+) before a 2-h continuous uptake with a mAb anti-Tac (in red) as well as the immunofluorescent distribution by using a polyclonal anti-TGN38 lumenal domain (in green). In the latter two panels, the nuclei are also stained with 4,6-diamidino-2-phenylindole (in blue). Transfected cells are marked with arrows. Experiment  : INS39 cells or Syn6t-10 cells were transfected, induced with ponasterone, and labeled as in experiment
: INS39 cells or Syn6t-10 cells were transfected, induced with ponasterone, and labeled as in experiment  , except that the cells were examined by confocal fluorescence microscopy.
, except that the cells were examined by confocal fluorescence microscopy.

Similar articles
-
Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles.J Cell Biol. 1998 Apr 20;141(2):359-71. doi: 10.1083/jcb.141.2.359. J Cell Biol. 1998. PMID: 9548715 Free PMC article.
-
MARCH-II is a syntaxin-6-binding protein involved in endosomal trafficking.Mol Biol Cell. 2005 Apr;16(4):1696-710. doi: 10.1091/mbc.e04-03-0216. Epub 2005 Feb 2. Mol Biol Cell. 2005. PMID: 15689499 Free PMC article.
-
Differential roles of syntaxin 7 and syntaxin 8 in endosomal trafficking.Mol Biol Cell. 1999 Nov;10(11):3891-908. doi: 10.1091/mbc.10.11.3891. Mol Biol Cell. 1999. PMID: 10564279 Free PMC article.
-
Maturing secretory granules: Where secretory and endocytic pathways converge.Adv Biol Regul. 2021 May;80:100807. doi: 10.1016/j.jbior.2021.100807. Epub 2021 Mar 25. Adv Biol Regul. 2021. PMID: 33866198 Review.
-
Secretory granule biogenesis: rafting to the SNARE.Trends Cell Biol. 2001 Mar;11(3):116-22. doi: 10.1016/s0962-8924(00)01907-3. Trends Cell Biol. 2001. PMID: 11306272 Review.
Cited by
-
Circadian Genes as Therapeutic Targets in Pancreatic Cancer.Front Endocrinol (Lausanne). 2020 Sep 11;11:638. doi: 10.3389/fendo.2020.00638. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33042011 Free PMC article. Review.
-
Regulation of intracellular membrane trafficking and cell dynamics by syntaxin-6.Biosci Rep. 2012 Aug;32(4):383-91. doi: 10.1042/BSR20120006. Biosci Rep. 2012. PMID: 22489884 Free PMC article. Review.
-
Circadian genes and insulin exocytosis.Cell Logist. 2011 Jan;1(1):32-36. doi: 10.4161/cl.1.1.14426. Cell Logist. 2011. PMID: 21686102 Free PMC article.
-
Secretion of soluble vascular endothelial growth factor receptor 1 (sVEGFR1/sFlt1) requires Arf1, Arf6, and Rab11 GTPases.PLoS One. 2012;7(9):e44572. doi: 10.1371/journal.pone.0044572. Epub 2012 Sep 4. PLoS One. 2012. PMID: 22962618 Free PMC article.
-
Insulin-like growth factor 1 (IGF-1) enhances the protein expression of CFTR.PLoS One. 2013;8(3):e59992. doi: 10.1371/journal.pone.0059992. Epub 2013 Mar 28. PLoS One. 2013. PMID: 23555857 Free PMC article.
References
-
- Advani, R.J., Bae, H.R., Bock, J.B., Chao, D.S., Doung, Y.C., Prekeris, R., Yoo, J.S., and Scheller, R.H. (1998). Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J. Biol. Chem. 273, 10317-10324. - PubMed
-
- Banting, G., and Ponnambalam, S. (1997). TGN38 and its orthologues: roles in post-TGN vesicle formation and maintenance of TGN morphology. Biochim. Biophys. Acta Mol. Cell Res. 1355, 209-217. - PubMed
-
- Bock, J.B., Lin, R.C., and Scheller, R.H. (1996). A new syntaxin family member implicated in targeting of intracellular transport vesicles. J. Biol. Chem. 271, 17961-17965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

