Identification of a novel sequence in PDZ-RhoGEF that mediates interaction with the actin cytoskeleton
- PMID: 14742719
- PMCID: PMC379273
- DOI: 10.1091/mbc.e03-07-0527
Identification of a novel sequence in PDZ-RhoGEF that mediates interaction with the actin cytoskeleton
Abstract
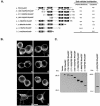
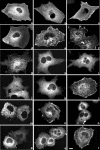
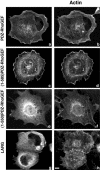
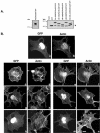
Small GTPases of the Rho family are crucial regulators of actin cytoskeleton rearrangements. Rho is activated by members of the Rho guanine-nucleotide exchange factor (GEF) family; however, mechanisms that regulate RhoGEFs are not well understood. This report demonstrates that PDZ-RhoGEF, a member of a subfamily of RhoGEFs that contain regulator of G protein signaling domains, is partially localized at or near the plasma membranes in 293T, COS-7, and Neuro2a cells, and this localization is coincident with cortical actin. Disruption of the cortical actin cytoskeleton in cells by using latrunculin B prevents the peri-plasma membrane localization of PDZ-RhoGEF. Coimmunoprecipitation and F-actin cosedimentation assays demonstrate that PDZ-RhoGEF binds to actin. Extensive deletion mutagenesis revealed the presence of a novel 25-amino acid sequence in PDZ-RhoGEF, located at amino acids 561-585, that is necessary and sufficient for localization to the actin cytoskeleton and interaction with actin. Last, PDZ-RhoGEF mutants that fail to interact with the actin cytoskeleton display enhanced Rho-dependent signaling compared with wild-type PDZ-RhoGEF. These results identify interaction with the actin cytoskeleton as a novel function for PDZ-RhoGEF, thus implicating actin interaction in organizing PDZ-RhoGEF signaling.
Figures





References
-
- Arai, A., Spencer, J.A., and Olson, E.N. (2002). STARS, a striated muscle activator of Rho signaling and serum response factor-dependent transcription. J. Biol. Chem. 277, 24453-24459. - PubMed
-
- Ausubel, F.M., Brent, R.E., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. (1992). Short Protocols in Molecular Biology. John Wiley & Sons: New York.
-
- Bellanger, J.M., Astier, C., Sardet, C., Ohta, Y., Stossel, T.P., and Debant, A. (2000). The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat. Cell Biol. 2, 888-892. - PubMed
-
- Bhattacharyya, R., and Wedegaertner, P.B. (2000). Galpha 13 requires palmitoylation for plasma membrane localization, Rho-dependent signaling, and promotion of p115-RhoGEF membrane binding. J. Biol. Chem. 275, 14992-14999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

