Apoplastic synthesis of nitric oxide by plant tissues
- PMID: 14742874
- PMCID: PMC341907
- DOI: 10.1105/tpc.017822
Apoplastic synthesis of nitric oxide by plant tissues
Abstract
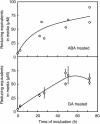
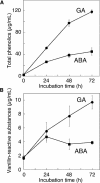
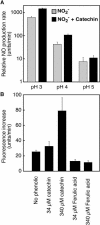
Nitric oxide (NO) is an important signaling molecule in animals and plants. In mammals, NO is produced from Arg by the enzyme NO synthase. In plants, NO synthesis from Arg using an NO synthase-type enzyme and from nitrite using nitrate reductase has been demonstrated previously. The data presented in this report strongly support the hypothesis that plant tissues also synthesize NO via the nonenzymatic reduction of apoplastic nitrite. As measured by mass spectrometry or an NO-reactive fluorescent probe, Hordeum vulgare (barley) aleurone layers produce NO rapidly when nitrite is added to the medium in which they are incubated. NO production requires an acid apoplast and is accompanied by a loss of nitrite from the medium. Phenolic compounds in the medium can increase the rate of NO production. The possible significance of apoplastic NO production for germinating grain and for plant roots is discussed.
Figures
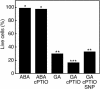
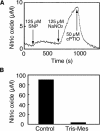
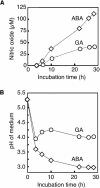
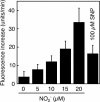


References
-
- Aastrup, S., Outtrup, H., and Erdal, K. (1984). Location of the proanthocyanidins in the barley grain. Carlsberg Res. Commun. 49, 105–109.
-
- Beligni, M.V., and Lamattina, L. (2001). Nitric oxide in plants: The history is just beginning. Plant Cell Environ. 24, 267–278.
-
- Berkels, R., Purol-Schnabel, S., and Roesen, R. (2001). A new method to measure nitrate/nitrite with a NO-sensitive electrode. J. Appl. Physiol. 90, 317–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

