Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases
- PMID: 14743497
- PMCID: PMC7167900
- DOI: 10.1002/path.1510
Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases
Abstract
Severe acute respiratory syndrome (SARS) is a new human infectious disease with significant morbidity and mortality. The disease has been shown to be associated with a new coronavirus (SARS-CoV). The clinical and epidemiological aspects of SARS have been described. Moreover, the viral genome of SARS-CoV has been fully sequenced. However, much of the biological behaviour of the virus is not known and data on the tissue and cellular tropism of SARS-CoV are limited. In this study, six fatal cases of SARS were investigated for the tissue and cellular tropism of SARS-CoV using an in-situ hybridization (ISH) technique. Among all the tissues studied, positive signals were seen in pneumocytes in the lungs and surface enterocytes in the small bowel. Infected pneumocytes were further confirmed by immunofluorescence-fluorescence in-situ hybridization (FISH) analysis. These results provide important information concerning the tissue tropism of SARS-CoV, which is distinct from previously identified human coronaviruses, and suggest the possible involvement of novel receptors in this infection. Whereas the lung pathology was dominated by diffuse alveolar damage, the gut was relatively intact. These findings indicated that tissue responses to SARS-CoV infection are distinct in different organs.
Copyright 2004 John Wiley & Sons, Ltd.
Figures
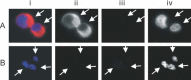



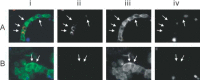
Comment in
-
Re: To KF, Tong JH, Chan PK, et al. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J Pathol 2004; 202: 157-163.J Pathol. 2004 Jun;203(2):729-30; author reply 730-1. doi: 10.1002/path.1575. J Pathol. 2004. PMID: 15141389 Free PMC article. No abstract available.
References
-
- World Health Organization . Severe acute respiratory syndrome (SARS). Multi‐countries outbreak update 73. http://www.who.int/csr/don/2003_06_04/en/. Access date 4 June 2003.
-
- Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1967–1976. - PubMed
-
- Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1953–1966. - PubMed
-
- World Health Organization . Recommendations for laboratories testing for SARS. http://www.who.int/csr/sars/labmethods/en/#lab. Access date 21 July 2003.
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

