Hypocretin (orexin): role in normal behavior and neuropathology
- PMID: 14744212
- PMCID: PMC8765219
- DOI: 10.1146/annurev.psych.55.090902.141545
Hypocretin (orexin): role in normal behavior and neuropathology
Abstract
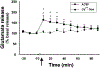
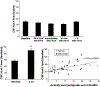
The hypocretins (Hcrts, also known as orexins) are two peptides, both synthesized by a small group of neurons, most of which are in the lateral hypothalamic and perifornical regions of the hypothalamus. The hypothalamic Hcrt system directly and strongly innervates and potently excites noradrenergic, dopaminergic, serotonergic, histaminergic, and cholinergic neurons. Hcrt also has a major role in modulating the release of glutamate and other amino acid transmitters. Behavioral investigations have revealed that Hcrt is released at high levels in active waking and rapid eye movement (REM) sleep and at minimal levels in non-REM sleep. Hcrt release in waking is increased markedly during periods of increased motor activity relative to levels in quiet, alert waking. Evidence for a role for Hcrt in food intake regulation is inconsistent. I hypothesize that Hcrt's major role is to facilitate motor activity tonically and phasically in association with motivated behaviors and to coordinate this facilitation with the activation of attentional and sensory systems. Degeneration of Hcrt neurons or genetic mutations that prevent the normal synthesis of Hcrt or of its receptors causes human and animal narcolepsy. Narcolepsy is characterized by an impaired ability to maintain alertness for long periods and by sudden losses of muscle tone (cataplexy). Administration of Hcrt can reverse symptoms of narcolepsy in animals, may be effective in treating human narcolepsy, and may affect a broad range of motivated behaviors.
Figures

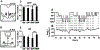

References
-
- Abrahamson EE, Moore RY. 2001. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 889:1–22 - PubMed
-
- Aldrich MS. 1998. Diagnostic aspects of narcolepsy. Neurology 50:S2–7 - PubMed
-
- Allen RP, Mignot E, Ripley B, Nishino S, Earley CJ. 2002. Increased CSF hypocretin-1 (orexin-A) in restless legs syndrome. Neurology 59:639–41 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

