Microfluidic approaches to malaria detection
- PMID: 14744562
- PMCID: PMC2726249
- DOI: 10.1016/j.actatropica.2003.11.009
Microfluidic approaches to malaria detection
Abstract
Microfluidic systems are under development to address a variety of medical problems. Key advantages of micrototal analysis systems based on microfluidic technology are the promise of small size and the integration of sample handling and measurement functions within a single, automated device having low mass-production costs. Here, we review the spectrum of methods currently used to detect malaria, consider their advantages and disadvantages, and discuss their adaptability towards integration into small, automated micro total analysis systems. Molecular amplification methods emerge as leading candidates for chip-based systems because they offer extremely high sensitivity, the ability to recognize malaria species and strain, and they will be adaptable to the detection of new genotypic signatures that will emerge from current genomic-based research of the disease. Current approaches to the development of chip-based molecular amplification are considered with special emphasis on flow-through PCR, and we present for the first time the method of malaria specimen preparation by dielectrophoretic field-flow-fractionation. Although many challenges must be addressed to realize a micrototal analysis system for malaria diagnosis, it is concluded that the potential benefits of the approach are well worth pursuing.
Figures

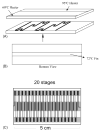


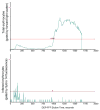

Similar articles
-
High-throughput screening approaches and combinatorial development of biomaterials using microfluidics.Acta Biomater. 2016 Apr 1;34:1-20. doi: 10.1016/j.actbio.2015.09.009. Epub 2015 Sep 8. Acta Biomater. 2016. PMID: 26361719 Review.
-
Handheld Purification-Free Nucleic Acid Testing Device for Point-of-Need Detection of Malaria from Whole Blood.ACS Sens. 2023 Feb 24;8(2):673-683. doi: 10.1021/acssensors.2c02169. Epub 2023 Jan 25. ACS Sens. 2023. PMID: 36696460 Free PMC article.
-
Automated quantitative cytological analysis using portable microfluidic microscopy.J Biophotonics. 2016 Jun;9(6):586-95. doi: 10.1002/jbio.201500108. Epub 2015 May 20. J Biophotonics. 2016. PMID: 25990413
-
Paper-based microfluidics for DNA diagnostics of malaria in low resource underserved rural communities.Proc Natl Acad Sci U S A. 2019 Mar 12;116(11):4834-4842. doi: 10.1073/pnas.1812296116. Epub 2019 Feb 19. Proc Natl Acad Sci U S A. 2019. PMID: 30782834 Free PMC article.
-
PCR microfluidic devices for DNA amplification.Biotechnol Adv. 2006 May-Jun;24(3):243-84. doi: 10.1016/j.biotechadv.2005.10.002. Epub 2005 Dec 2. Biotechnol Adv. 2006. PMID: 16326063 Review.
Cited by
-
Current and developing technologies for monitoring agents of bioterrorism and biowarfare.Clin Microbiol Rev. 2005 Oct;18(4):583-607. doi: 10.1128/CMR.18.4.583-607.2005. Clin Microbiol Rev. 2005. PMID: 16223949 Free PMC article. Review.
-
Removal of malaria-infected red blood cells using magnetic cell separators: A computational study.Appl Math Comput. 2012 Feb 15;218(12):6841-6850. doi: 10.1016/j.amc.2011.12.057. Appl Math Comput. 2012. PMID: 22345827 Free PMC article.
-
Effect of input voltage frequency on the distribution of electrical stresses on the cell surface based on single-cell dielectrophoresis analysis.Sci Rep. 2020 Jan 9;10(1):68. doi: 10.1038/s41598-019-56952-4. Sci Rep. 2020. PMID: 31919394 Free PMC article.
-
Insulator-based dielectrophoresis of mitochondria.Biomicrofluidics. 2014 Mar 3;8(2):021801. doi: 10.1063/1.4866852. eCollection 2014 Mar. Biomicrofluidics. 2014. PMID: 24959306 Free PMC article.
-
Separation of platelets from other blood cells in continuous-flow by dielectrophoresis field-flow-fractionation.Biomicrofluidics. 2011 Sep;5(3):34122-341228. doi: 10.1063/1.3640045. Epub 2011 Sep 21. Biomicrofluidics. 2011. PMID: 22662047 Free PMC article.
References
-
- Aceti A, Bonincontro A, Cametti C, Celestino D, Leri O. Electrical conductivity of human erythrocytes infected with Plasmodium falciparum and its modification following quinine therapy. Trans R Soc Trop Med Hyg. 1990;84 (5):671–672. - PubMed
-
- Aubouy A, Jafari S, Huart V, Migot-Nabias F, Mayombo J, Durand R, Bakary M, Le Bras J, Deloron P. DHFR and DHPS genotypes of Plasmodium falciparum isolates from Gabon correlate with in vitro activity of pyrimethamine and cycloguanil, but not with sulfadoxine–pyrimethamine treatment efficacy. J Antimicrob Chemother. 2003;52 (1):43–49. - PubMed
-
- Bangs MJ, Rusmiarto S, Gionar YR, Chan AS, Dave K, Ryan JR. Evaluation of a dipstick malaria sporozoite panel assay for detection of naturally infected mosquitoes. J Med Entomol. 2002;39 (2):324–330. - PubMed
-
- Barkan D, Ginsburg H, Golenser J. Optimisation of flow cytometric measurement of parasitaemia in plasmodium-infected mice. Int J Parasitol. 2000;30 (5):649–653. - PubMed
-
- Barker RH, Jr, Banchongaksorn T, Courval JM, Suwonkerd W, Rimwungtragoon K, Wirth DF. Plasmodium falciparum and P. vivax: factors affecting sensitivity and specificity of PCR-based diagnosis of malaria. Exp Parasitol. 1994;79 (1):41–49. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

