Asymmetric division and lineage commitment at the level of hematopoietic stem cells: inference from differentiation in daughter cell and granddaughter cell pairs
- PMID: 14744992
- PMCID: PMC2211802
- DOI: 10.1084/jem.20030929
Asymmetric division and lineage commitment at the level of hematopoietic stem cells: inference from differentiation in daughter cell and granddaughter cell pairs
Abstract
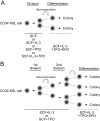
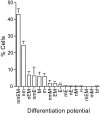
How hematopoietic stem cells (HSCs) commit to a particular lineage is unclear. A high degree of HSC purification enabled us to address this issue at the clonal level. Single-cell transplantation studies revealed that 40% of the CD34-/low, c-Kit+, Sca-1+, and lineage marker- (CD34-KSL) cells in adult mouse bone marrow were able, as individual cells, to reconstitute myeloid and B- and T-lymphoid lineages over the long-term. Single-cell culture showed that >40% of CD34-KSL cells could form neutrophil (n)/macrophage (m)/erythroblast (E)/megakaryocyte (M) (nmEM) colonies. Assuming that a substantial portion of long-term repopulating cells can be detected as nmEM cells within this population, we compared differentiation potentials between individual pairs of daughter and granddaughter cells derived in vitro from single nmEM cells. One of the two daughter or granddaughter cells remained an nmEM cell. The other showed a variety of combinations of differentiation potential. In particular, an nmEM cell directly gave rise, after one cell division, to progenitor cells committed to nm, EM, or M lineages. The probability of asymmetric division of nmEM cells depended on the cytokines used. These data strongly suggest that lineage commitment takes place asymmetrically at the level of HSCs under the influence of external factors.
Figures

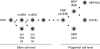
References
-
- Lansdorp, P.M. 1997. Self-renewal of stem cells. Biol. Blood Marrow Transplant. 3:171–178. - PubMed
-
- Metcalf, D. 1998. Lineage commitment and maturation in hematopoietic cells: The case for extrinsic regulation. Blood. 92:354–352. - PubMed
-
- Ogawa, M. 1999. Stochastic model revisited. Int. J. Hematol. 69:2–5. - PubMed
-
- Weissman, I.L. 2000. Stem cells: Units of development, units of regeneration, and units in evolution. Cell. 100:157–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

