CD1d-dependent activation of NKT cells aggravates atherosclerosis
- PMID: 14744994
- PMCID: PMC2211791
- DOI: 10.1084/jem.20030997
CD1d-dependent activation of NKT cells aggravates atherosclerosis
Abstract
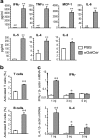
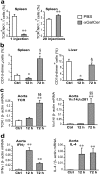
Adaptive and innate immunity have been implicated in the pathogenesis of atherosclerosis. Given their abundance in the lesion, lipids might be targets of the atherosclerosis-associated immune response. Natural killer T (NKT) cells can recognize lipid antigens presented by CD1 molecules. We have explored the role of CD1d-restricted NKT cells in atherosclerosis by using apolipoprotein E-deficient (apoE-/-) mice, a hypercholesterolemic mouse model that develops atherosclerosis. ApoE-/- mice crossed with CD1d-/- (CD1d-/-apoE-/-) mice exhibited a 25% decrease in lesion size compared with apoE-/- mice. Administration of alpha-galactosylceramide, a synthetic glycolipid that activates NKT cells via CD1d, induced a 50% increase in lesion size in apoE-/- mice, whereas it did not affect lesion size in apoE-/-CD1d-/- mice. Treatment was accompanied by an early burst of cytokines (IFNgamma, MCP-1, TNFalpha, IL-2, IL-4, IL-5, and IL-6) followed by sustained increases in IFNgamma and IL-4 transcripts in the spleen and aorta. Early activation of both T and B cells was followed by recruitment of T and NKT cells to the aorta and activation of inflammatory genes. These results show that activation of CD1d-restricted NKT cells exacerbates atherosclerosis.
Figures
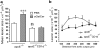
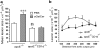

References
-
- Binder, C.J., M.K. Chang, P.X. Shaw, Y.I. Miller, K. Hartvigsen, A. Dewan, and J.L. Witztum. 2002. Innate and acquired immunity in atherogenesis. Nat. Med. 8:1218–1226. - PubMed
-
- Hansson, G.K. 2001. Immune mechanisms in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 21:1876–1890. - PubMed
-
- Breslow, J.L. 1996. Mouse models of atherosclerosis. Science. 272:685–688. - PubMed
-
- Joyce, S., and L. Van Kaer. 2003. CD1-restricted antigen presentation: an oily matter. Curr. Opin. Immunol. 15:95–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

