Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans
- PMID: 14745002
- PMCID: PMC2172228
- DOI: 10.1083/jcb.200311112
Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans
Abstract
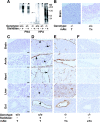
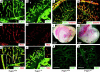
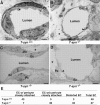
The core 1 beta1-3-galactosyltransferase (T-synthase) transfers Gal from UDP-Gal to GalNAcalpha1-Ser/Thr (Tn antigen) to form the core 1 O-glycan Galbeta1-3GalNAcalpha1-Ser/Thr (T antigen). The T antigen is a precursor for extended and branched O-glycans of largely unknown function. We found that wild-type mice expressed the NeuAcalpha2-3Galbeta1-3GalNAcalpha1-Ser/Thr primarily in endothelial, hematopoietic, and epithelial cells during development. Gene-targeted mice lacking T-synthase instead expressed the nonsialylated Tn antigen in these cells and developed brain hemorrhage that was uniformly fatal by embryonic day 14. T-synthase-deficient brains formed a chaotic microvascular network with distorted capillary lumens and defective association of endothelial cells with pericytes and extracellular matrix. These data reveal an unexpected requirement for core 1-derived O-glycans during angiogenesis.
Copyright The Rockefeller University Press
Figures




References
-
- Berger, E.G. 1999. Tn-syndrome. Biochim. Biophys. Acta. 1455:255–268. - PubMed
-
- Blixt, O., B.E. Collins, I.M. Van Den Nieuwenhof, P.R. Crocker, and J.C. Paulson. 2003. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J. Biol. Chem. 278:31007–31019. - PubMed
-
- Brockhausen, I. 1999. Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta. 1473:67–95. - PubMed
-
- Brockhausen, I., J. Yang, N. Dickinson, S. Ogata, and S.H. Itzkowitz. 1998. Enzymatic basis for sialyl-Tn expression in human colon cancer cells. Glycoconj. J. 15:595–603. - PubMed
-
- Bugge, T.H., Q. Xiao, K.W. Kombrinck, M.J. Flick, K. Holmback, M.J. Danton, M.C. Colbert, D.P. Witte, K. Fujikawa, E.W. Davie, and J.L. Degen. 1996. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc. Natl. Acad. Sci. USA. 93:6258–6263. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

