Coat protein regulates formation of replication complexes during tobacco mosaic virus infection
- PMID: 14745003
- PMCID: PMC337067
- DOI: 10.1073/pnas.0307778101
Coat protein regulates formation of replication complexes during tobacco mosaic virus infection
Abstract
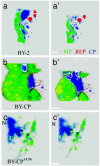
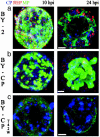
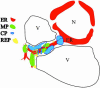
The genome of tobacco mosaic virus (TMV) encodes replicase protein(s), movement protein (MP), and capsid protein (CP). On infection, one or more viral proteins direct the assembly of virus replication complexes (VRCs), in association with host-derived membranes. The impact of CP-mediated resistance on the structures of the replication complexes was examined in nontransgenic and transgenic BY-2 cell lines that produce wild-type CP, mutant CP(T42W), and Ds-Red, which was targeted to endoplasmic reticulum by using immunofluorescence and 3D microscopy. We developed a model of VRCs that shows a clear association of MP with and surrounding the endoplasmic reticulum. Replicase is located within the MP bodies, as well as isolated sites throughout the cell. CP surrounds the VRCs. CP enhances the production of MP and increases the size of the VRC; however, the mutant CP(T42W) reduces the amount of MP and interferes with the formation of VRCs. We propose a regulatory role of the CP in the establishment of the VRC. We suggest that the lack of formation of VRCs restricts the efficiency of virus replication and the formation of virus movement complexes, resulting in restriction of cell-cell spread of infection. This results in higher levels of plant CP-mediated protection provided by CP(T42W).
Figures



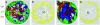
References
-
- Noueiry, A. O. & Ahlquist, P. (2003) Annu. Rev. Phytopathol. 41, 77–98. - PubMed
-
- Ivanowski, D. (1903) Z. Pflanzenkr. Pflanzenschutz 13, 1–41.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

