A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis
- PMID: 14745012
- PMCID: PMC337052
- DOI: 10.1073/pnas.0308114100
A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis
Abstract
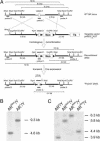
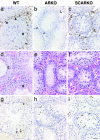
Androgens control spermatogenesis, but germ cells themselves do not express a functional androgen receptor (AR). Androgen regulation is thought to be mediated by Sertoli and peritubular myoid cells, but their relative roles and the mechanisms involved remain largely unknown. Using Cre/loxP technology, we have generated mice with a ubiquitous knockout of the AR as well as mice with a selective AR knockout in Sertoli cells (SC) only. Mice with a floxed exon 2 of the AR gene were crossed with mice expressing Cre recombinase ubiquitously or selectively in SC (under control of the anti-Müllerian hormone gene promoter). AR knockout males displayed a complete androgen insensitivity phenotype. Testes were located abdominally, and germ cell development was severely disrupted. In contrast, SC AR knockout males showed normal testis descent and development of the male urogenital tract. Expression of the homeobox gene Pem, which is androgen-regulated in SC, was severely decreased. Testis weight was reduced to 28% of that in WT littermates. Stereological analysis indicated that the number of SC was unchanged, whereas numbers of spermatocytes, round spermatids, and elongated spermatids were reduced to 64%, 3%, and 0% respectively of WT. These changes were associated with increased germ cell apoptosis and grossly reduced expression of genes specific for late spermatocyte or spermatid development. It is concluded that cell-autonomous action of the AR in SC is an absolute requirement for androgen maintenance of complete spermatogenesis, and that spermatocyte/spermatid development/survival critically depends on androgens.
Figures

References
-
- Sharpe, R. M. (1994) in The Physiology of Reproduction, eds. Knobil, E. & Neill, J. D. (Raven, New York), pp. 1363–2434.
-
- Weinbauer, G. F. & Nieschlag, E. (1998) in Testosterone: Action–Deficiency, Substitution, eds. Nieschlag, E. & Behre, H. M. (Springer, Berlin), pp. 143–168.
-
- McLachlan, R. I., O'Donnell, L., Meachem, S. J., Stanton, P. G., de Kretser, D. M., Pratis, K. & Robertson, D. M. (2002) Recent Prog. Horm. Res. 57, 149–179. - PubMed
-
- Singh, J., O'Neill, C. & Handelsman, D. J. (1995) Endocrinology 136, 5311–5321. - PubMed
-
- Bremner, W. J., Millar, M. R., Sharpe, R. M. & Saunders, P. T. (1994) Endocrinology 135, 1227–1234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

