Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing
- PMID: 14745017
- PMCID: PMC337056
- DOI: 10.1073/pnas.0308308100
Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing
Abstract
Homology-dependent RNA silencing occurs in many eukaryotic cells. We reported recently that nodaviral infection triggers an RNA silencing-based antiviral response (RSAR) in Drosophila, which is capable of a rapid virus clearance in the absence of expression of a virus-encoded suppressor. Here, we present further evidence to show that the Drosophila RSAR is mediated by the RNA interference (RNAi) pathway, as the viral suppressor of RSAR inhibits experimental RNAi initiated by exogenous double-stranded RNA and RSAR requires the RNAi machinery. We demonstrate that RNAi also functions as a natural antiviral immunity in mosquito cells. We further show that vaccinia virus and human influenza A, B, and C viruses each encode an essential protein that suppresses RSAR in Drosophila. The vaccinia and influenza viral suppressors, E3L and NS1, are distinct double-stranded RNA-binding proteins and essential for pathogenesis by inhibiting the mammalian IFN-regulated innate antiviral response. We found that the double-stranded RNA-binding domain of NS1, implicated in innate immunity suppression, is both essential and sufficient for RSAR suppression. These findings provide evidence that mammalian virus proteins can inhibit RNA silencing, implicating this mechanism as a nucleic acid-based antiviral immunity in mammalian cells.
Figures


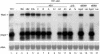
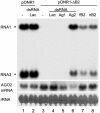

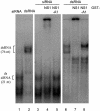
References
-
- Denli, A. M. & Hannon, G. J. (2003) Trends Biochem. Sci. 28, 196–201. - PubMed
-
- Hamilton, A. J. & Baulcombe, D. C. (1999) Science 286, 950–952. - PubMed
-
- Hammond, S. M., Bernstein, E., Beach, D. & Hannon, G. J. (2000) Nature 404, 293–296. - PubMed
-
- Zamore, P. D., Tuschl, T., Sharp, P. A. & Bartel, D. P. (2000) Cell 101, 25–33. - PubMed
-
- Bernstein, E., Caudy, A. A., Hammond, S. M. & Hannon, G. J. (2001) Nature 409, 363–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

