Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D
- PMID: 14745018
- PMCID: PMC341775
- DOI: 10.1073/pnas.0308319100
Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D
Erratum in
- Proc Natl Acad Sci U S A. 2006 May 23;103(21):8298
Abstract
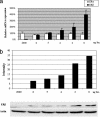
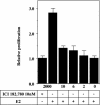
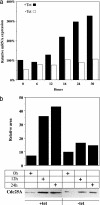
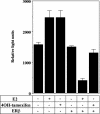
Estrogen receptor (ER) beta counteracts the activity of ERalpha in many systems. In agreement with this, we show in this study that induced expression of ERbeta in the breast cancer cell line T47D reduces 17beta-estradiol-stimulated proliferation when expression of ERbeta mRNA equals that of ERalpha. Induction of ERbeta reduces growth of exponentially proliferating cells with a concomitant decrease in components of the cell cycle associated with proliferation, namely cyclin E, Cdc25A (a key regulator of Cdk2), p45(Skp2) (a key regulator of p27(Kip1) proteolysis), and an increase in the Cdk inhibitor p27(Kip1). We also observed a reduced Cdk2 activity. These findings suggest a possible role for ERbeta in breast cancer and imply that ERbeta-specific ligands may reduce proliferation of ER-positive breast cancer cells through actions on the G(1) phase cell-cycle machinery.
Figures



References
-
- Liu, M. M., Albanese, C., Anderson, C. M., Hilty, K., Webb, P., Uht, R. M., Price, R. H., Jr., Pestell, R. G. & Kushner, P. J. (2002) J. Biol. Chem. 277, 24353-24360. - PubMed
-
- Nelson, H. D., Humphrey, L. L., Nygren, P., Teutsch, S. M. & Allan, J. D. (2002) J. Am. Med. Assoc. 288, 872-881. - PubMed
-
- Lin, C. Q., Singh, J., Murata, K., Itahana, Y., Parrinello, S., Liang, S. H., Gillett, C. E., Campisi, J. & Desprez, P. Y. (2000) Cancer Res. 60, 1332-1340. - PubMed
-
- Strom, A., Arai, N., Leers, J. & Gustafsson, J.-Å. (2000) Oncogene 19, 5951-5953. - PubMed
-
- Dubik, D., Dembinski, T. C. & Shiu, R. P. (1987) Cancer Res. 47, 65117-65121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

