Proton transfer dynamics at the membrane/water interface: dependence on the fixed and mobile pH buffers, on the size and form of membrane particles, and on the interfacial potential barrier
- PMID: 14747306
- PMCID: PMC1303918
- DOI: 10.1016/S0006-3495(04)74146-6
Proton transfer dynamics at the membrane/water interface: dependence on the fixed and mobile pH buffers, on the size and form of membrane particles, and on the interfacial potential barrier
Abstract
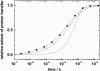
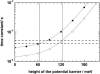
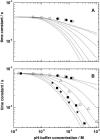
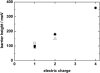
Crossing the membrane/water interface is an indispensable step in the transmembrane proton transfer. Elsewhere we have shown that the low dielectric permittivity of the surface water gives rise to a potential barrier for ions, so that the surface pH can deviate from that in the bulk water at steady operation of proton pumps. Here we addressed the retardation in the pulsed proton transfer across the interface as observed when light-triggered membrane proton pumps ejected or captured protons. By solving the system of diffusion equations we analyzed how the proton relaxation depends on the concentration of mobile pH buffers, on the surface buffer capacity, on the form and size of membrane particles, and on the height of the potential barrier. The fit of experimental data on proton relaxation in chromatophore vesicles from phototropic bacteria and in bacteriorhodopsin-containing membranes yielded estimates for the interfacial potential barrier for H(+)/OH(-) ions of approximately 120 meV. We analyzed published data on the acceleration of proton equilibration by anionic pH buffers and found that the height of the interfacial barrier correlated with their electric charge ranging from 90 to 120 meV for the singly charged species to >360 meV for the tetra-charged pyranine.
Figures


References
-
- Arata, H., I. Takenaka, and M. Nishimura. 1987. Flash-induced proton release in Rhodopseudomonas sphaeroides spaeroplasts. J. Biochem. 101:261–265. - PubMed
-
- Baur, J. E., and R. M. Wightman. 1991. Diffusion-coefficients determined with microelectrodes. J. Electroanal. Chem. 305:73–81.
-
- Bell, R. P. 1973. The Proton in Chemistry. Chapman & Hall, London, UK.
-
- Berry, R. S., S. A. Rice, and J. Ross. 1980. Physical Chemistry. Wiley, New York.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

