Inner field compensation as a tool for the characterization of asymmetric membranes and Peptide-membrane interactions
- PMID: 14747327
- PMCID: PMC1303939
- DOI: 10.1016/S0006-3495(04)74167-3
Inner field compensation as a tool for the characterization of asymmetric membranes and Peptide-membrane interactions
Abstract
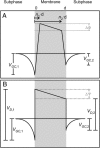
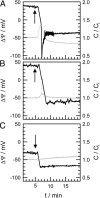
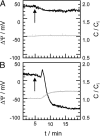
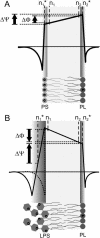
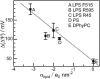
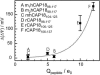
Symmetric and asymmetric planar lipid bilayers prepared according to the Montal-Mueller method are a powerful tool to characterize peptide-membrane interactions. Several electrical properties of lipid bilayers such as membrane current, membrane capacitance, and the inner membrane potential differences and their changes can be deduced. The time-resolved determination of peptide-induced changes in membrane capacitance and inner membrane potential difference are of high importance for the characterization of peptide-membrane interactions. Intercalation and accumulation of peptides lead to changes in membrane capacitance, and membrane interaction of charged peptides induces changes in the charge distribution within the membrane and with that to changes in the membrane potential profile. In this study, we establish time-resolved measurements of the capacitance minimization potential DeltaPsi on various asymmetric planar lipid bilayers using the inner field compensation method. The results are compared to the respective ones of inner membrane potential differences DeltaPhi determined from ion carrier transport measurements. Finally, the time courses of membrane capacitances and of DeltaPsi have been used to characterize the interaction of cathelicidins with reconstituted lipid matrices of various Gram-negative bacteria.
Figures

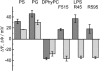
Similar articles
-
Interaction of CAP18-derived peptides with membranes made from endotoxins or phospholipids.Biophys J. 2001 Jun;80(6):2935-45. doi: 10.1016/S0006-3495(01)76259-5. Biophys J. 2001. PMID: 11371466 Free PMC article.
-
Electrochemical screening of anti-microbial peptide LL-37 interaction with phospholipids.Bioelectrochemistry. 2007 May;70(2):205-13. doi: 10.1016/j.bioelechem.2006.07.006. Epub 2006 Jul 12. Bioelectrochemistry. 2007. PMID: 16949887
-
Magainin 2 channel formation in planar lipid membranes: the role of lipid polar groups and ergosterol.Eur Biophys J. 2003 Mar;32(1):22-32. doi: 10.1007/s00249-002-0262-y. Epub 2002 Nov 22. Eur Biophys J. 2003. PMID: 12632203
-
Role of Lipid Composition, Physicochemical Interactions, and Membrane Mechanics in the Molecular Actions of Microbial Cyclic Lipopeptides.J Membr Biol. 2019 Jun;252(2-3):131-157. doi: 10.1007/s00232-019-00067-4. Epub 2019 May 16. J Membr Biol. 2019. PMID: 31098678 Review.
-
The interaction of antimicrobial peptides with membranes.Adv Colloid Interface Sci. 2017 Sep;247:521-532. doi: 10.1016/j.cis.2017.06.001. Epub 2017 Jun 5. Adv Colloid Interface Sci. 2017. PMID: 28606715 Review.
Cited by
-
Modeling the electrostatic potential of asymmetric lipopolysaccharide membranes: the MEMPOT algorithm implemented in DelPhi.J Comput Chem. 2014 Jul 15;35(19):1418-1429. doi: 10.1002/jcc.23632. Epub 2014 May 6. J Comput Chem. 2014. PMID: 24799021 Free PMC article.
-
Native Pig Neutrophil Products: Insights into Their Antimicrobial Activity.Microorganisms. 2023 Aug 20;11(8):2119. doi: 10.3390/microorganisms11082119. Microorganisms. 2023. PMID: 37630679 Free PMC article.
-
Liposome Deformation Induced by Membrane-Binding Peptides.Micromachines (Basel). 2023 Feb 2;14(2):373. doi: 10.3390/mi14020373. Micromachines (Basel). 2023. PMID: 36838073 Free PMC article.
-
Influence of Membrane Asymmetry on OmpF Insertion, Orientation and Function.Membranes (Basel). 2023 May 16;13(5):517. doi: 10.3390/membranes13050517. Membranes (Basel). 2023. PMID: 37233578 Free PMC article.
-
Protein reconstitution into freestanding planar lipid membranes for electrophysiological characterization.Nat Protoc. 2015 Jan;10(1):188-98. doi: 10.1038/nprot.2015.003. Epub 2014 Dec 31. Nat Protoc. 2015. PMID: 25551663
References
-
- Babakov, A. V., L. N. Ermishkin, and E. A. Liberman. 1966. Influence of electric field on the capacity of phospholipid membranes. Nature. 210:953–955. - PubMed
-
- Boggs, J. M., and G. Rangaraj. 1985. Phase transitions and fatty acid spin label behavior in interdigitated lipid phases induced by glycerol and polymyxin. Biochim. Biophys. Acta. 816:221–233. - PubMed
-
- Carius, W. 1976. Voltage dependence of bilayer membrane capacitance—harmonic response to ac excitation with dc bias. J. Colloid Interface Sci. 57:301–307.
-
- Cevc, G. 1990. Membrane electrostatics. Biochim. Biophys. Acta. 1031:311–382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

