Response of equatorial x-ray reflections and stiffness to altered sarcomere length and myofilament lattice spacing in relaxed skinned cardiac muscle
- PMID: 14747335
- PMCID: PMC1303893
- DOI: 10.1016/S0006-3495(04)74175-2
Response of equatorial x-ray reflections and stiffness to altered sarcomere length and myofilament lattice spacing in relaxed skinned cardiac muscle
Abstract
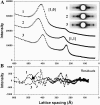
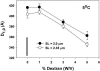
Low angle x-ray diffraction measurements of myofilament lattice spacing (D(1,0)) and equatorial reflection intensity ratio (I(1,1)/I(1,0)) were made in relaxed skinned cardiac trabeculae from rats. We tested the hypothesis that the degree of weak cross-bridge (Xbr) binding, which has been shown to be obligatory for force generation in skeletal muscle, is modulated by changes in lattice spacing in skinned cardiac muscle. Altered weak Xbr binding was detected both by changes in I(1,1)/I(1,0) and by measurements of chord stiffness (chord K). Both measurements showed that, similar to skeletal muscle, the probability of weak Xbr binding at 170-mM ionic strength was significantly enhanced by lowering temperature to 5 degrees C. The effects of lattice spacing on weak Xbr binding were therefore determined under these conditions. Changes in D(1,0), I(1,1)/I(1,0), and chord K by osmotic compression with dextran T500 were determined at sarcomere lengths (SL) of 2.0 and 2.35 micro m. At each SL increasing [dextran] caused D(1,0) to decrease and both I(1,1)/I(1,0) and chord K to increase, indicating increased weak Xbr binding. The results suggest that in intact cardiac muscle increasing SL and decreasing lattice spacing could lead to increased force by increasing the probability of initial weak Xbr binding.
Figures




References
-
- Brenner, B. 1986. The cross-bridge cycle in muscle. Mechanical, biochemical, and structural studies on single skinned rabbit psoas fibers to characterize cross-bridge kinetics in muscle for correlation with the actomyosin-ATPase in solution. Basic Res. Cardiol. 81:1–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

