Surface functionalized cationic lipid-DNA complexes for gene delivery: PEGylated lamellar complexes exhibit distinct DNA-DNA interaction regimes
- PMID: 14747350
- PMCID: PMC1303908
- DOI: 10.1016/S0006-3495(04)74190-9
Surface functionalized cationic lipid-DNA complexes for gene delivery: PEGylated lamellar complexes exhibit distinct DNA-DNA interaction regimes
Abstract
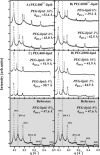
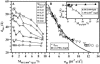
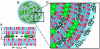
Cationic lipid-DNA (CL-DNA) complexes are abundantly used in nonviral gene therapy clinical applications. Surface functionality is the next step in developing these complexes as competent, target-specific gene carriers. Poly(ethylene glycol) (PEG) is the natural choice to serve as a protective coat or act as a tether for a specific ligand on the surface of these complexes due to its biocompatibility and ability to convey stealth-like properties. Understanding the effect of PEG on the internal structure and surface properties of CL-DNA complexes is essential in developing vectors with more complex derivatives of PEG, such as Arg-Gly-Asp (RGD)-based peptide-PEG-lipids. We report on x-ray diffraction studies to probe the internal structure of CL-DNA complexes consisting of a ternary mixture of cationic lipids, neutral lipids, and PEG-lipids. The PEG-coated complexes are found to exhibit a structure consistent with the lamellar phase. In addition, three distinct DNA interchain interaction regimes were found to exist, due to a), repulsive long-range electrostatic forces; b), short-range repulsive hydration forces; and c), novel polymer-induced depletion attraction forces in two dimensions. Optical microscopy and reporter gene assays further demonstrate the incorporation of the PEG-lipids into the lamellar CL-DNA complexes under biologically relevant conditions, revealing surface modification. Both techniques show that PEG-lipids with a polymer chain of molecular weight 400 do not provide adequate shielding of the PEGylated CL-DNA complexes, whereas PEG-lipids with a polymer chain of molecular weight 2000 confer stealth-like properties. This surface functionalization is a crucial initial step in the development of competent vectors for in vivo systemic gene delivery and suggests that a second type of surface functionality can be added specifically for targeting by the incorporation of peptide-PEG-lipids.
Figures




References
-
- Allen, T. M. 1994. Long-circulating (sterically stabilized) liposomes for targeted drug-delivery. Trends Pharmacol. Sci. 15:215–220. - PubMed
-
- Bradley, A. J., D. V. Devine, S. M. Ansell, J. Janzen, and D. E. Brooks. 1998. Inhibition of liposome-induced complement activation by incorporated poly(ethylene glycol) lipids. Arch. Biochem. Biophys. 357:185–194. - PubMed
-
- Bruinsma, R., and J. Mashl. 1998. Long-range electrostatic interaction in DNA cationic lipid complexes. Europhys. Lett. 41:165–170.
-
- Chesnoy, S., and L. Huang. 2000. Structure and function of lipid-DNA complexes for gene delivery. Annu. Rev. Biophys. Biomol. Struct. 29:27–47. - PubMed
-
- Devanand, K., and J. C. Selser. 1991. Asymptotic behavior and long-range interactions in aqueous solutions of poly(ethylene oxide). Macromolecules. 24:5943–5947.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

