The influenza B virus nonstructural NS1 protein is essential for efficient viral growth and antagonizes beta interferon induction
- PMID: 14747551
- PMCID: PMC369500
- DOI: 10.1128/jvi.78.4.1865-1872.2004
The influenza B virus nonstructural NS1 protein is essential for efficient viral growth and antagonizes beta interferon induction
Abstract
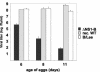
We analyzed the functions of the influenza B virus nonstructural NS1-B protein, both by utilizing a constructed mutant virus (Delta NS1-B) lacking the NS1 gene and by testing the activities of the protein when expressed in cells. The mutant virus replicated to intermediate levels in 6-day-old embryonated chicken eggs that contain an immature interferon (IFN) system, whereas older eggs did not support viral propagation to a significant extent. The Delta NS1-B virus was a substantially stronger inducer of beta IFN (IFN-beta) transcripts in human lung epithelial cells than the wild type, and furthermore, transiently expressed NS1-B protein efficiently inhibited virus-dependent activation of the IFN-beta promoter. Interestingly, replication of the Delta NS1-B knockout virus was attenuated by more than 4 orders of magnitude in tissue culture cells containing or lacking functional IFN-alpha/beta genes. These findings show that the NS1-B protein functions as a viral IFN antagonist and indicate a further requirement of this protein for efficient viral replication that is unrelated to blocking IFN effects.
Figures


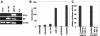
References
-
- Bandyopadhyay, S. K., G. T. Leonard, Jr., T. Bandyopadhyay, G. R. Stark, and G. C. Sen. 1995. Transcriptional induction by double-stranded RNA is mediated by interferon-stimulated response elements without activation of interferon-stimulated gene factor 3. J. Biol. Chem. 270:19624-19629. - PubMed
-
- Barber, G. N. 2000. The interferons and cell death: guardians of the cell or accomplices of apoptosis? Semin. Cancer Biol. 10:103-111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

