The K1 protein of Kaposi's sarcoma-associated herpesvirus activates the Akt signaling pathway
- PMID: 14747556
- PMCID: PMC369501
- DOI: 10.1128/jvi.78.4.1918-1927.2004
The K1 protein of Kaposi's sarcoma-associated herpesvirus activates the Akt signaling pathway
Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) has been implicated in Kaposi's sarcoma, as well as in primary effusion lymphoma and multicentric Castleman's disease. The K1 protein of KSHV has been shown to induce cellular transformation and focus formation and to deregulate B-lymphocyte signaling pathways by functionally mimicking the activated B-cell receptor complex. Here we show that expression of K1 in B lymphocytes targets the phosphatidylinositol-3 kinase pathway, leading to the activation of the Akt kinase and the inhibition of the phosphatase PTEN. We also demonstrate that activation of Akt by the K1 protein leads to the phosphorylation and inhibition of members of the forkhead (FKHR) transcription factor family, which are key regulators of cell cycle progression and apoptosis. We demonstrate that K1 can inhibit apoptosis induced by the FKHR proteins and by stimulation of the Fas receptor. Our observations suggest that the K1 viral protein promotes cell survival pathways and may contribute to KSHV pathogenesis by preventing virally infected cells from undergoing apoptosis prematurely.
Figures



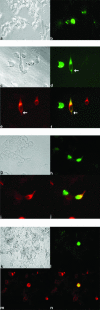

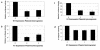

References
-
- Alam, M. K., S. Davison, N. Siddiqui, J. D. Norton, and J. J. Murphy. 1997. Ectopic expression of Bcl-2, but not Bcl-xL, rescues Ramos B cells from Fas-mediated apoptosis. Eur. J. Immunol. 27:3485-3491. - PubMed
-
- Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr. Biol. 7:261-269. - PubMed
-
- Andjelkovic, M., D. R. Alessi, R. Meier, A. Fernandez, N. J. Lamb, M. Frech, P. Cron, P. Cohen, J. M. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515-31524. - PubMed
-
- Bais, C., A. Van Geelen, P. Eroles, A. Mutlu, C. Chiozzini, S. Dias, R. L. Silverstein, S. Rafii, and E. A. Mesri. 2003. Kaposi's sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/ KDR. Cancer Cell 3:131-143. - PubMed
-
- Beitz, L. O., D. A. Fruman, T. Kurosaki, L. C. Cantley, and A. M. Scharenberg. 1999. SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J. Biol. Chem. 274:32662-32666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

