Distribution of hydrophobic residues is crucial for the fusogenic properties of the Ebola virus GP2 fusion peptide
- PMID: 14747578
- PMCID: PMC369453
- DOI: 10.1128/jvi.78.4.2131-2136.2004
Distribution of hydrophobic residues is crucial for the fusogenic properties of the Ebola virus GP2 fusion peptide
Abstract
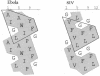
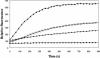
The lipid-destabilizing properties of the N-terminal domain of the GP2 of Ebola virus were investigated. Our results suggest that the domain of Ebola virus needed for fusion is shorter than that previously reported. The fusogenic properties of this domain are related to its oblique orientation at the lipid/water interface owing to an asymmetric distribution of the hydrophobic residues when helical.
Figures


References
-
- Atherton, E., C. J. Logan, and R. C. Sheppard. 1981. Peptide synthesis. II. Procedures for solid-phase synthesis by N-fluorenylmethoxycarbonylamino-acids on polyamide supports: synthesis of substance P and of acyl carrier protein 65-74 decapeptide. J. Chem. Soc. Perkin Trans. I 1:538.
-
- Bradshaw, J. P., M. J. Darkes, T. A. Harroun, J. Katsaras, and R. M. Epand. 2000. Oblique membrane insertion of viral fusion peptide probed by neutron diffraction. Biochemistry 39:6581-6585. - PubMed
-
- Brasseur, R. 1991. Differentiation of lipid-associating helices by use of three-dimensional molecular hydrophobicity potential calculations. J. Biol. Chem. 266:16120-16127. - PubMed
-
- Brasseur, R., T. Pillot, L. Lins, J. Vandekerckhove, and M. Rosseneu. 1997. Peptides in membranes: tipping the balance of membrane stability. Trends Biochem. Sci. 22:167-171. - PubMed
-
- Brasseur, R. 2000. Tilted peptides: a motif for membrane destabilization (hypothesis). Mol. Membr. Biol. 17:31-40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

