Centrosome maturation: measurement of microtubule nucleation throughout the cell cycle by using GFP-tagged EB1
- PMID: 14747658
- PMCID: PMC341778
- DOI: 10.1073/pnas.0308205100
Centrosome maturation: measurement of microtubule nucleation throughout the cell cycle by using GFP-tagged EB1
Abstract
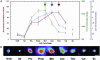
Understanding how cells regulate microtubule nucleation during the cell cycle has been limited by the inability to directly observe nucleation from the centrosome. To view nucleation in living cells, we imaged GFP-tagged EB1, a microtubule tip-binding protein, and determined rates of nucleation by counting the number of EB1-GFP comets emerging from the centrosome over time. Nucleation rate increased 4-fold between G(2) and prophase and continued to rise through anaphase and telophase, reaching a maximum of 7 times interphase rates. We tested several models for centrosome maturation, including gamma-tubulin recruitment and increased centrosome size. The centrosomal concentration of gamma-tubulin reached a maximum at metaphase, and centrosome size increased through anaphase, whereas nucleation remained high through telophase, implying the presence of additional regulatory processes. Injection of anti-gamma-tubulin antibodies significantly blocked nucleation during metaphase but was less effective during anaphase, suggesting that a nucleation mechanism independent of gamma-tubulin contributes to centrosome function after metaphase.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

