Comparative population pharmacokinetics of lorazepam and midazolam during long-term continuous infusion in critically ill patients
- PMID: 14748812
- PMCID: PMC1884441
- DOI: 10.1046/j.1365-2125.2003.01957.x
Comparative population pharmacokinetics of lorazepam and midazolam during long-term continuous infusion in critically ill patients
Abstract
Aims: It is well established that there is a wide intra- and interindividual variability in dose requirements for lorazepam and midazolam in intensive care patients. The objective of this study was to compare the population pharmacokinetics of lorazepam and midazolam after long-term continuous infusion in mechanically ventilated critically ill patients.
Methods: Forty-nine critically ill patients randomly received either lorazepam (n = 28) or midazolam (n = 21) by continuous infusion for at least 24 h. Multiple blood samples were obtained for determination of the drug and metabolite concentrations by HPLC. Population pharmacokinetic models were developed using the Non-Linear Mixed Effect Modelling (NONMEM) program. The influence of selected covariates was investigated. The prospective performance of the models was evaluated on the basis of results in separate groups of patients for lorazepam (n = 31) and midazolam (n = 33).
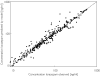
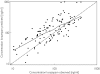
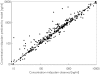
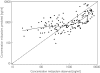
Results: The pharmacokinetics of lorazepam were best described by a two-compartment model. Alcohol abuse, positive end expiratory pressure (PEEP) and age were identified as significant covariates. Total body clearance for patients without alcohol abuse was 4.13 - (PEEP - 5) x 0.42 l h-1, and 0.74 l h-1 for patients with alcohol abuse. The volume of distribution was 0.74 l, the steady state volume of distribution was 56 - (age - 58) x 2.1 l and the intercompartmental clearance was 10 l h-1. The proportional residual error was 15% and the median absolute prediction error was 13.6% with a bias of 1.5%. The pharmacokinetics of midazolam were best described by a two-compartment model with alcohol abuse, APACHE score and age as significant covariates. Total body clearance for patients without alcohol abuse was 11.3 - (age - 57) x 0.14 l h-1, and 7.27 - (age -57) x 0.14 l h-1 for patients with alcohol abuse. The volume of distribution was 7.15 l, the steady state volume of distribution was 431 l, and the intercompartmental clearance was 40.8 - (APACHE score - 26) x 2.75 l h-1. The proportional residual error was 31% with an additive residual error of 32 ng ml-1. The median absolute prediction error was 12.9% with a bias of 1.2%. The prospective performance in the lorazepam evaluation group was better with the covariate adjusted model, but in the midazolam evaluation group it was not better than with the simple model. In all models a tendency to overestimate the lower plasma concentrations was observed.
Conclusions: The pharmacokinetics of both lorazepam and midazolam were well described by a two-compartment model. Inclusion of alcohol abuse and age as covariates improved both models. PEEP was identified as an additional covariate for lorazepam, and the APACHE score for midazolam. For both drugs there is a large interindividual variability in their pharmacokinetics when used for long-term sedation in critically ill patients. However, the intra-individual variability is much lower for lorazepam.
Figures
Similar articles
-
Population pharmacodynamic modelling of lorazepam- and midazolam-induced sedation upon long-term continuous infusion in critically ill patients.Eur J Clin Pharmacol. 2006 Mar;62(3):185-94. doi: 10.1007/s00228-005-0085-8. Epub 2006 Jan 20. Eur J Clin Pharmacol. 2006. PMID: 16425056 Clinical Trial.
-
A double-blind, randomized comparison of i.v. lorazepam versus midazolam for sedation of ICU patients via a pharmacologic model.Anesthesiology. 2001 Aug;95(2):286-98. doi: 10.1097/00000542-200108000-00007. Anesthesiology. 2001. PMID: 11506097 Clinical Trial.
-
Population pharmacokinetics of lorazepam and midazolam and their metabolites in intensive care patients on continuous venovenous hemofiltration.Am J Kidney Dis. 2005 Feb;45(2):360-71. doi: 10.1053/j.ajkd.2004.09.004. Am J Kidney Dis. 2005. PMID: 15685515
-
Altered Pharmacokinetics in Prolonged Infusions of Sedatives and Analgesics Among Adult Critically Ill Patients: A Systematic Review.Clin Ther. 2018 Sep;40(9):1598-1615.e2. doi: 10.1016/j.clinthera.2018.07.021. Epub 2018 Aug 31. Clin Ther. 2018. PMID: 30173953
-
Optimal intravenous dosing strategies for sedatives and analgesics in the intensive care unit.Crit Care Clin. 1995 Oct;11(4):827-47. Crit Care Clin. 1995. PMID: 8535981 Review.
Cited by
-
Lumping of physiologically-based pharmacokinetic models and a mechanistic derivation of classical compartmental models.J Pharmacokinet Pharmacodyn. 2010 Aug;37(4):365-405. doi: 10.1007/s10928-010-9165-1. Epub 2010 Jul 27. J Pharmacokinet Pharmacodyn. 2010. PMID: 20661651
-
Sedation strategy and ICU delirium: a multicentre, population-based propensity score-matched cohort study.BMJ Open. 2021 Jul 20;11(7):e045087. doi: 10.1136/bmjopen-2020-045087. BMJ Open. 2021. PMID: 34285003 Free PMC article.
-
Pharmacokinetics Alterations of Midazolam Infusion versus Bolus Administration in Mechanically Ventilated Critically Ill Patients.Iran J Pharm Res. 2013 Spring;12(2):483-8. Iran J Pharm Res. 2013. PMID: 24250625 Free PMC article.
-
Outcomes in Critically Ill Patients Sedated with Intravenous Lormetazepam or Midazolam: A Retrospective Cohort Study.J Clin Med. 2021 Sep 10;10(18):4091. doi: 10.3390/jcm10184091. J Clin Med. 2021. PMID: 34575204 Free PMC article.
-
Population Pharmacokinetics of Fentanyl in the Critically Ill.Crit Care Med. 2016 Jan;44(1):64-72. doi: 10.1097/CCM.0000000000001347. Crit Care Med. 2016. PMID: 26491862 Free PMC article.
References
-
- Burns AM, Shelly MP, Park GR. The use of sedative agents in critically ill patients. Drugs. 1992;43:507–15. - PubMed
-
- Aitkenhead AR. Analgesia and sedation in intensive care. Br J Anaesth. 1989;63:196–206. - PubMed
-
- Swart EL, Strack van Schijndel RJM, van Loenen AC, Thijs LG. Continuous infusion of lorazepam vs midazolam in patients in the intensive care unit: sedation with lorazepam is easier to manage and is more cost-effective. Crit Care Med. 1999;27:263–9. - PubMed
-
- Mandema JW, Tuk B, van Steveninck AL, Breimer DD, Cohen AF, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the central nervous system effects of midazolam and its main metabolite α-hydroxymidazolam in healthy volunteers. Clin Pharmacol Ther. 1992;51:715–28. - PubMed
-
- Bauer TM, Ritz R, Haberthür C, Riem Ha H, Hunkeler W, Sleight AJ, Scollo-Lavizzari G, Haefeli WE. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet. 1995;346:145–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

