Population pharmacokinetics of artemether and dihydroartemisinin following single intramuscular dosing of artemether in African children with severe falciparum malaria
- PMID: 14748813
- PMCID: PMC1884434
- DOI: 10.1046/j.1365-2125.2003.01986.x
Population pharmacokinetics of artemether and dihydroartemisinin following single intramuscular dosing of artemether in African children with severe falciparum malaria
Abstract
Aims: To determine the population pharmacokinetics of artemether and dihydroartemisinin in African children with severe malaria and acidosis associated with respiratory distress following an intramuscular injection of artemether.
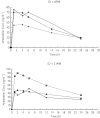
Methods: Following a single intramuscular (i.m.) injection of 3.2 mg kg-1 artemether, blood samples were withdrawn at various times over 24 h after the dose. Plasma was assayed for artemether and dihydroartemisinin by gas chromatography-mass spectrometry. The software program NONMEM was used to fit the concentration-time data and investigate the influence of a range of clinical characteristics (respiratory distress and metabolic acidosis, demographic features and disease) on the pharmacokinetics of artemether and dihydroartemisinin.
Results: A total of 100 children with a median age of 36.4 (range 5-108) months were recruited into the study and data from 90 of these children (30 with respiratory distress and 60 with no respiratory distress) were used in the population pharmacokinetic analysis. The best model to describe the disposition of artemether was a one-compartment model with first-order absorption and elimination. The population estimate of clearance (clearance/bioavailability, CL/F) was 14.3 l h-1 with 53% intersubject variability and that of the terminal half-life was 18.5 h. If it was assumed that artemisin displays "flip-flop" kinetics, the elimination half-life was estimated to be 21 min and the corresponding volume of distribution was 8.44 l, with an intersubject variability of 104%. None of the covariates could be identified as having any influence on the disposition of artemether. The disposition of dihydroartemisinin was fitted separately using a one-compartment linear model in which the volume of distribution was fixed to the same value as that of artemether. Assuming that artemether is completely converted to dihydroartemisinin, the estimated value of CL/F for dihydroartemisinin was 93.5 l h-1, with an intersubject variability of 90.2%. The clearance of dihydroartemisinin was formation rate limited.
Conclusions: Administration of a single 3.2 mg kg-1 i.m. dose of artemether to African children with severe malaria and acidosis is characterized by variable absorption kinetics, probably related to drug formulation characteristics rather than to pathophysiological factors. Use of i.m. artemether in such children needs to be reconsidered.
Figures



References
-
- Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404. - PubMed
-
- Waller D, Krishna S, Crawley J, et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis. 1995;21:577–87. - PubMed
-
- Taylor TE, Borgstein A, Molyneux ME. Acid-base status in paediatric Plasmodium falciparum malaria. Q J Med. 1993;86:99–109. - PubMed
-
- Kildeberg P. Metabolic acidosis in infantile gastroenteritis. Acta Paedtr Scand. 1965;54:155–67. - PubMed
-
- Murphy SA, Mberu E, Muhia D, et al. The disposition of intramuscular artemether in children with cerebral malaria: a preliminary study. Trans R Soc Trop Med Hyg. 1997;91:331–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

