Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4
- PMID: 14749364
- PMCID: PMC344190
- DOI: 10.1128/MCB.24.4.1464-1469.2004
Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4
Abstract
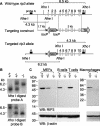
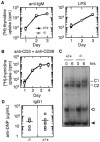
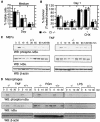
RIP3 is a member of the RIP kinase family. It is expressed in the embryo and in multiple adult tissues, including most hemopoietic cell lineages. Several studies have implicated RIP3 in the regulation of apoptosis and NF-kappa B signaling, but whether RIP3 promotes or attenuates activation of the NF-kappa B family of transcription factors has been controversial. We have generated RIP3-deficient mice by gene targeting and find RIP3 to be dispensable for normal mouse development. RIP3-deficient cells showed normal sensitivity to a variety of apoptotic stimuli and were indistinguishable from wild-type cells in their ability to activate NF-kappa B signaling in response to the following: human tumor necrosis factor (TNF), which selectively engages mouse TNF receptor 1; cross-linking of the B- or T-cell antigen receptors; peptidoglycan, which activates Toll-like receptor 2; and lipopolysaccharide (LPS), which stimulates Toll-like receptor 4. Consistent with these observations, RIP3-deficient mice exhibited normal antibody production after immunization with a T-dependent antigen and normal interleukin-1 beta (IL-1 beta), IL-6, and TNF production after LPS treatment. Thus, we can exclude RIP3 as an essential modulator of NF-kappa B signaling downstream of several receptor systems.
Figures


References
-
- Devin, A., A. Cook, Y. Lin, Y. Rodriguez, M. Kelliher, and Z. Liu. 2000. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity 12:419-429. - PubMed
-
- Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. - PubMed
-
- Karin, M. 1999. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene 18:6867-6874. - PubMed
-
- Kasof, G. M., J. C. Prosser, D. Liu, M. V. Lorenzi, and B. C. Gomes. 2000. The RIP-like kinase, RIP3, induces apoptosis and NF-kappaB nuclear translocation and localizes to mitochondria. FEBS Lett. 473:285-291. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
