Regulation of apoptosis by the Ft1 protein, a new modulator of protein kinase B/Akt
- PMID: 14749367
- PMCID: PMC344167
- DOI: 10.1128/MCB.24.4.1493-1504.2004
Regulation of apoptosis by the Ft1 protein, a new modulator of protein kinase B/Akt
Abstract
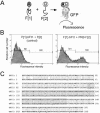
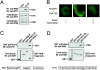
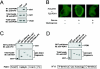
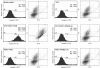
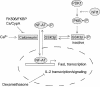
The serine/threonine kinase protein kinase B (PKB)/Akt plays a central role in many cellular processes, including cell growth, glucose metabolism, and apoptosis. However, the identification and validation of novel regulators or effectors is key to future advances in understanding the multiple functions of PKB. Here we report the identification of a novel PKB binding protein, called Ft1, from a cDNA library screen using a green fluorescent protein-based protein-fragment complementation assay. We show that the Ft1 protein interacts directly with PKB, enhancing the phosphorylation of both of its regulatory sites by promoting its interaction with the upstream kinase PDK1. Further, the modulation of PKB activity by Ft1 has a strong effect on the apoptosis susceptibility of T lymphocytes treated with glucocorticoids. We demonstrate that this phenomenon occurs via a PDK1/PKB/GSK3/NF-ATc signaling cascade that controls the production of the proapoptotic hormone Fas ligand. The wide distribution of Ft1 in adult tissues suggests that it could be a general regulator of PKB activity in the control of differentiation, proliferation, and apoptosis in many cell types.
Figures


References
-
- Alberola-Ila, J., S. Takaki, J. D. Kerner, and R. M. Perlmutter. 1997. Differential signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 15:125-154. - PubMed
-
- Alessi, D. R., M. Deak, A. Casamayor, F. B. Caudwell, N. Morrice, D. G. Norman, P. Gaffney, C. B. Reese, C. N. MacDougall, D. Harbison, A. Ashworth, and M. Bownes. 1997. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol. 7:776-789. - PubMed
-
- Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7:261-269. - PubMed
-
- Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930-1934. - PubMed
-
- Belham, C., S. Wu, and J. Avruch. 1999. Intracellular signalling: PDK1—a kinase at the hub of things. Curr. Biol. 9:R93-R96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
