Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins
- PMID: 14749368
- PMCID: PMC344170
- DOI: 10.1128/MCB.24.4.1505-1515.2004
Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins
Abstract
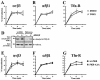
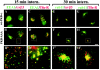
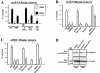
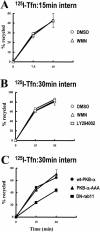
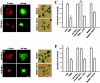
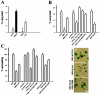
Protein kinase B (PKB)/Akt is known to promote cell migration, and this may contribute to the enhanced invasiveness of malignant cells. To elucidate potential mechanisms by which PKB/Akt promotes the migration phenotype, we have investigated its role in the endosomal transport and recycling of integrins. Whereas the internalization of alpha v beta 3 and alpha 5 beta 1 integrins and their transport to the recycling compartment were independent of PKB/Akt, the return of these integrins (but not internalized transferrin) to the plasma membrane was regulated by phosphatidylinositol 3-kinases and PKB/Akt. The blockade of integrin recycling and cell spreading on integrin ligands effected by inhibition of PKB/Akt was reversed by inhibition of glycogen synthase kinase 3 (GSK-3). Moreover, expression of nonphosphorylatable active GSK-3 beta mutant GSK-3 beta-A9 suppressed recycling of alpha 5 beta 1 and alpha v beta 3 and reduced cell spreading on ligands for these integrins, indicating that PKB/Akt promotes integrin recycling by phosphorylating and inactivating GSK-3. We propose that the ability of PKB/Akt to act via GSK-3 to promote the recycling of matrix receptors represents a key mechanism whereby integrin function and cell migration can be regulated by growth factors.
Figures

References
-
- Bakkeren, D. L., C. M. de Jeu-Jaspars, M. J. Kroos, and H. G. van Eijk. 1987. Release of iron from endosomes is an early step in the transferrin cycle. Int. J. Biochem. 19:179-186. - PubMed
-
- Balendran, A., R. M. Biondi, P. C. Cheung, A. Casamayor, M. Deak, and D. R. Alessi. 2000. A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase Cζ (PKCζ) and PKC-related kinase 2 by PDK1. J. Biol. Chem. 275:20806-20813. - PubMed
-
- Barbieri, M. A., A. D. Kohn, R. A. Roth, and P. D. Stahl. 1998. Protein kinase B/akt and rab5 mediate Ras activation of endocytosis. J. Biol. Chem. 273:19367-19370. - PubMed
-
- Byzova, T. V., C. K. Goldman, N. Pampori, K. A. Thomas, A. Bett, S. J. Shattil, and E. F. Plow. 2000. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell 6:851-860. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
