Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death
- PMID: 14749373
- PMCID: PMC344198
- DOI: 10.1128/MCB.24.4.1570-1581.2004
Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death
Abstract
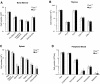
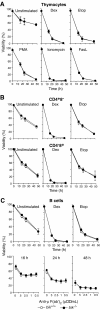
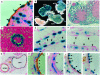
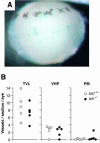
The BH3-only members of the Bcl-2 protein family are essential for initiation of programmed cell death and stress-induced apoptosis. We have determined the expression pattern in mice of the BH3-only protein Bik, also called Blk or Nbk, and examined its physiological function by gene targeting. We found that Bik is expressed widely in the hematopoietic compartment and in endothelial cells of the venous but not arterial lineages. Nevertheless, its loss did not increase the numbers of such cells in mice or protect hematopoietic cells in vitro from apoptosis induced by cytokine withdrawal or diverse other cytotoxic stimuli. Moreover, whereas loss of the BH3-only protein Bim rescued mice lacking the prosurvival protein Bcl-2 from fatal polycystic kidney disease and lymphopenia, loss of Bik did not. These results indicate that any function of Bik in programmed cell death and stress-induced apoptosis must overlap that of other BH3-only proteins.
Figures




References
-
- Adams, J. M. 2003. Ways of dying: multiple pathways to apoptosis. Genes Dev. 17:2481-2495. - PubMed
-
- Amanna, I. J., K. Clise-Dwyer, F. E. Nashold, K. A. Hoag, and C. E. Hayes. 2001. Cutting edge: A/WySnJ transitional B cells overexpress the chromosome 15 proapoptotic Blk gene and succumb to premature apoptosis. J. Immunol. 167:6069-6072. - PubMed
-
- Bouillet, P., S. Cory, L.-C. Zhang, A. Strasser, and J. M. Adams. 2001. Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim. Dev. Cell 1:645-653. - PubMed
-
- Bouillet, P., D. Metcalf, D. C. S. Huang, D. M. Tarlinton, T. W. H. Kay, F. Köntgen, J. M. Adams, and A. Strasser. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286:1735-1738. - PubMed
-
- Bouillet, P., J. F. Purton, D. I. Godfrey, L.-C. Zhang, L. Coultas, H. Puthalakath, M. Pellegrini, S. Cory, J. M. Adams, and A. Strasser. 2002. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415:922-926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
