Functional interaction between poly(ADP-Ribose) polymerase 2 (PARP-2) and TRF2: PARP activity negatively regulates TRF2
- PMID: 14749375
- PMCID: PMC344168
- DOI: 10.1128/MCB.24.4.1595-1607.2004
Functional interaction between poly(ADP-Ribose) polymerase 2 (PARP-2) and TRF2: PARP activity negatively regulates TRF2
Abstract
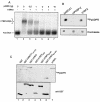
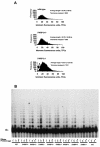
The DNA damage-dependent poly(ADP-ribose) polymerase-2 (PARP-2) is, together with PARP-1, an active player of the base excision repair process, thus defining its key role in genome surveillance and protection. Telomeres are specialized DNA-protein structures that protect chromosome ends from being recognized and processed as DNA strand breaks. In mammals, telomere protection depends on the T(2)AG(3) repeat binding protein TRF2, which has been shown to remodel telomeres into large duplex loops (t-loops). In this work we show that PARP-2 physically binds to TRF2 with high affinity. The association of both proteins requires the N-terminal domain of PARP-2 and the myb domain of TRF2. Both partners colocalize at promyelocytic leukemia bodies in immortalized telomerase-negative cells. In addition, our data show that PARP activity regulates the DNA binding activity of TRF2 via both a covalent heteromodification of the dimerization domain of TRF2 and a noncovalent binding of poly(ADP-ribose) to the myb domain of TRF2. PARP-2(-/-) primary cells show normal telomere length as well as normal telomerase activity compared to wild-type cells but display a spontaneously increased frequency of chromosome and chromatid breaks and of ends lacking detectable T(2)AG(3) repeats. Altogether, these results suggest a functional role of PARP-2 activity in the maintenance of telomere integrity.
Figures






References
-
- Ame, J. C., V. Rolli, V. Schreiber, C. Niedergang, F. Apiou, P. Decker, S. Muller, T. Hoger, J. Menissier-de Murcia, and G. de Murcia. 1999. PARP-2, a novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J. Biol. Chem. 274:17860-17868. - PubMed
-
- Ancelin, K., M. Brunori, S. Bauwens, C. E. Koering, C. Brun, M. Ricoul, J. P. Pommier, L. Sabatier, and E. Gilson. 2002. Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol. Cell. Biol. 22:3474-3487. - PMC - PubMed
-
- Augustin, A., C. Spenlehauer, H. Dumond, J. Menissier-De Murcia, M. Piel, A. C. Schmit, F. Apiou, J. L. Vonesch, M. Kock, M. Bornens, and G. De Murcia. 2003. PARP-3 localizes preferentially to the daughter centriole and interferes with the G1/S cell cycle progression. J. Cell Sci. 116:1551-1562. - PubMed
-
- Bailey, S. M., M. N. Cornforth, A. Kurimasa, D. J. Chen, and E. H. Goodwin. 2001. Strand-specific postreplicative processing of mammalian telomeres. Science 293:2462-2465. - PubMed
-
- Bianchi, A., and T. de Lange. 1999. Ku binds telomeric DNA in vitro. J. Biol. Chem. 274:21223-21227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
