Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function
- PMID: 14749421
- PMCID: PMC6729820
- DOI: 10.1523/JNEUROSCI.0949-03.2004
Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function
Abstract
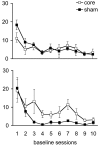
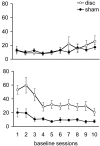
Anatomically segregated systems linking the frontal cortex and the striatum are involved in various aspects of cognitive, affective, and motor processing. In this study, we examined the effects of combined unilateral lesions of the medial prefrontal cortex (mPFC) and the core subregion of the nucleus accumbens (AcbC) in opposite hemispheres (disconnection) on a continuous performance, visual attention test [five-choice serial reaction-time task (5CSRTT)]. The disconnection lesion produced a set of specific changes in performance of the 5CSRTT, resembling changes that followed bilateral AcbC lesions while, in addition, comprising a subset of the behavioral changes after bilateral mPFC lesions previously reported using the same task. Specifically, both mPFC/AcbC disconnection and bilateral AcbC lesions markedly affected aspects of response control related to affective feedback, as indexed by perseverative responding in the 5CSRTT. These effects were comparable, although not identical, to those in animals with either bilateral AcbC or mPFC/AcbC disconnection lesions. The mPFC/AcbC disconnection resulted in a behavioral profile largely distinct from that produced by disconnection of a similar circuit described previously, between the mPFC and the dorsomedial striatum, which were shown to form a functional network underlying aspects of visual attention and attention to action. This distinction provides an insight into the functional specialization of corticostriatal circuits in similar behavioral contexts.
Figures





References
-
- Apicella P, Ljungberg T, Scarnati E, Schultz W (1991) Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res 85: 491–500. - PubMed
-
- Baunez C, Robbins TW (1999) Effects of dopamine depletion of the dorsal striatum and further interaction with subthalamic nucleus lesions in an attentional task in the rat. Neuroscience 92: 1343–1356. - PubMed
-
- Bowman EM, Brown VJ (1998) Effects of excitotoxic lesions of the rat ventral striatum on the perception of reward cost. Exp Brain Res 123: 439–448. - PubMed
-
- Bowman EM, Aigner TG, Richmond BJ (1996) Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J Neurophysiol 75: 1061–1073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
